Johns Hopkins University
BullFrog AI Holdings, Inc.
Exhibit 10.6
EXCLUSIVE LICENSE AGREEMENT
Johns Hopkins University and BullFrog AI Holdings, Inc.
JHU Agreement Number A40219
This AGREEMENT is entered into by and between the Johns Hopkins University (“JHU”), a Maryland corporation having an address at 3400 N. Charles Street, Baltimore, Maryland, 21218- 2695, and BullFrog AI Holdings, Inc., (“LICENSEE”), a Nevada corporation having an address at 325 Ellington Blvd., #317, Gaithersburg, MD 20878, and is effective on the 22nd day of February, 2022 (“EFFECTIVE DATE”).
RECITALS
WHEREAS, JHU owns, by assignment or otherwise from members of its faculty and staff, certain valuable inventions, know-how, and data as specified in Exhibit A-1, which JHU desires to have commercialized to make useful products and services available for the benefit of the public, including members of undeveloped countries and poor populations, as soon as possible, in accordance with JHU’s mission and purpose;
WHEREAS, LICENSEE and JHU entered into a Non-Disclosure Evaluation, and Option Agreement dated October 15, 2021 (“OPTION AGREEMENT”); and
WHEREAS, LICENSEE exercised the license option from JHU on November 22, 2021, and in accordance with the terms of the OPTION AGREEMENT, the parties now wish to enter into an exclusive license agreement; and
WHEREAS, LICENSEE desires to obtain certain rights in accordance with this AGREEMENT so that it may develop, manufacture, use and/or distribute certain products and services for public use and benefit as soon as possible.
NOW THEREFORE, the parties agree, with the intent to be legally bound, as follows:
| 1. | DEFINITIONS AND SCOPE |
Capitalized terms have the meanings provided by Exhibit B or as defined in the body of this AGREEMENT.
| 2. | GRANT OF LICENSES |
| 2.1. | Grant of Exclusive Patent License. Subject to this AGREEMENT, JHU grants LICENSEE an exclusive license under the LICENSED PATENTS to make, have made, use, import, export, offer to sell and sell LICENSED PRODUCTS in the LICENSED TERRITORY and FIELD OF USE and to grant SUBLICENSES subject to the limitations provided by this AGREEMENT. | |
| 2.2. | Grant of Non-Exclusive Right to Use Data, and Know-How. JHU grants LICENSEE a non-exclusive right to use the LICENSED DATA, or LICENSED KNOW-HOW, existing as of the EFFECTIVE DATE of this AGREEMENT and as identified in and subject to restrictions identified in Exhibit A-1. This right to use is granted solely to LICENSEE to permit LICENSEE to make, have made, use, import, export, offer to sell, sell, develop, and commercialize LICENSED PRODUCTS in the LICENSED TERRITORY in the FIELD OF USE, provided that LICENSEE may grant SUBLICENSES to LICENSED DATA and LICENSED KNOW-HOW, solely in connection with SUBLICENSES of the LICENSED PATENTS and solely to the extent needed to practice the LICENSED PATENTS and subject to the limitations provided by this AGREEMENT. |
| 1 |
| 2.3. | Affiliate Rights and Obligations. The LICENSED RIGHTS granted herein extend to AFFILIATES, except that AFFILIATES may not grant SUBLICENSES without JHU’s written consent. An AFFILIATE that exercises rights under this AGREEMENT shall automatically be bound by all terms and conditions of this AGREEMENT, including but not limited to indemnity and insurance provisions and the obligation to pay ROYALTIES. All acts or omissions by an AFFILIATE shall be considered acts or omissions of LICENSEE, which is, and shall remain, liable for them. | |
| 2.4. | Sublicense Notification. LICENSEE shall provide a complete and unredacted copy of each SUBLICENSE to JHU within thirty (30) days of execution. Each SUBLICENSE shall (i) expressly reference this AGREEMENT and declare void and unenforceable against JHU any terms contrary to this AGREEMENT; (ii) prohibit sublicensing by the SUBLICENSEE; (iii) expressly incorporate the Articles (inclusive of subsections) of this AGREEMENT numbered 4, 5, 6, 7, 8, 9, 10, 11, and 12 for the benefit of JHU; and (iv) acknowledge JHU as a third party beneficiary of the SUBLICENSE having the right to audit and enforce its terms and (v) expressly require SUBLICENSEE to provide LICENSEE diligence reports on an annual basis for the express purpose of providing those SUBLICENSEE diligence reports to JHU. In addition, each SUBLICENSE shall provide for its own immediate termination or expiration upon termination or expiration of this AGREEMENT, unless LICENSEE’s entire right and interest in such SUBLICENSE (including all rights to receive ROYALTIES and other payments) is assigned in writing to JHU with JHU’s consent, which shall not be unreasonably withheld or delayed. Failure to comply with the requirements of this Section 2.4 shall cause any purported SUBLICENSE to be void. | |
| 2.5. | Retained Research and Publication Rights. JHU retains the unrestricted right, on behalf of itself, its faculty and staff and non-profit academic or research institutions to whom JHU extends such rights, to practice and use any LICENSED RIGHTS described in Exhibit A-1 for any research or non-profit purpose, including sponsored research and collaborations with commercial entities and assessment and treatment of patients at Johns Hopkins Health System/JHU institutions. In addition, the right of JHU’s faculty and staff to publish all information concerning what is described in Exhibit A-1 shall not be restricted by this AGREEMENT. | |
| 2.6. | Government Rights. LICENSED PATENTS arising from research funded in whole or part by the United States government are subject to the Bayh Dole Act and its implementing regulations (35 U.S.C. §§ 200-204, 37 CFR Part 401) (collectively, “Bayh Dole Obligations”), including requirements to take effective steps in a reasonable time to achieve practical application of the LICENSED PATENTS in the FIELD OF USE and to assure LICENSED PRODUCTS sold or produced in the United States be “manufactured substantially in the United States.” LICENSEE shall comply with, and cooperate with, JHU in assuring compliance with the Bayh Dole Obligations. JHU’s obligations under Title 35 Sections 200-204 of the United States Code include the grant of an irrevocable, non-exclusive, nontransferable, royalty-free worldwide license to LICENSED PATENTS by JHU to the United States government, and a statement of United States government patent rights on all LICENSED PATENTS. All determinations of federal research funding involvement will be made solely by JHU, and JHU’s determination shall be honored by LICENSEE. |
| 2 |
| 2.7. | Humanitarian Rights and Obligations. |
| 2.7.1. | The parties will cooperate such that essential medicines developed under this AGREEMENT can be made available in LEAST DEVELOPED COUNTRIES. JHU agrees to consider reasonable requests of LICENSEE for a commensurate reduction of payment obligations to JHU to facilitate the availability of LICENSED PRODUCTS in such countries. | |
| 2.7.2. | Provided JHU first consults with LICENSEE, pursuant to this Section 2.7.2, JHU retains the right to grant rights to manufacture, use, distribute, sell and import the LICENSED RIGHTS described in Exhibit A-1 to a QUALIFIED HUMANITARIAN ORGANIZATION for HUMANITARIAN PURPOSES, provided that any such grant shall expressly prohibit the manufacture, use, distribution, sale or importation of any LICENSED PRODUCT in a country other than a LEAST DEVELOPED COUNTRY. Prior to granting such rights, JHU will notify LICENSEE, and LICENSEE shall have the first right to grant such rights to such QUALIFIED HUMANITARIAN ORGANIZATION. |
| 2.8. | Commercial Development Sublicenses. In the event LICENSEE is unable or unwilling to develop a LICENSED PRODUCT for an unserved market, use, indication or territory, upon JHU’s written request and LICENSEE’S failure to provide JHU with a reasonable development plan for such unserved market, use, indication, or territory within ninety (90) days of such written request, LICENSEE shall negotiate with one or more potential sublicensees identified by JHU to authorize development of such product. LICENSEE shall not, however, be obligated to enter into a sublicense that poses a material risk to the successful development and commercialization of LICENSED PRODUCTS by LICENSEE. | |
| 2.9. | Exclusions. Nothing in this AGREEMENT imposes obligations on JHU or grants rights in any JHU technology, intellectual property or other assets except as expressly identified in this AGREEMENT. Except as specifically provided in this AGREEMENT, JHU does not have any obligation to provide to LICENSEE any know how, inventions, data, materials, or assistance. |
| 3. | DILIGENCE AND DILIGENCE REPORTS |
| 3.1. | Milestones. LICENSEE shall achieve the MILESTONES set forth in Exhibit A-3 and shall notify JHU of the achievement of each MILESTONE within thirty (30) days of achieving them. | |
| 3.2. | Extension of Diligence Milestone. LICENSEE may request, in writing, an extension of the period for achieving a diligence MILESTONE set forth in Exhibit A-3 (each a MILESTONE) by up to six months. JHU will grant the requested extension provided (i) LICENSEE has diligently pursued achievement of the MILESTONE; and (ii) LICENSEE remits the milestone payment amount within thirty (30) days of achievement of the delayed MILESTONE. The extension of a MILESTONE shall automatically extend the deadline for subsequent MILESTONES of Exhibit A-3 respecting the same subject matter by like amount. LICENSEE may seek extensions for MILESTONES no more than twice during the term of this AGREEMENT. |
| 3 |
| 3.3. | Diligence Reports. Annually, on or before March 1 of each year, LICENSEE shall submit a Diligence Report for the prior calendar year to JHU substantially in the form attached as Exhibit D and in sufficient detail to facilitate JHU’s compliance with its Bayh Dole Obligations. |
| 4. | FEES, ROYALTIES, MILESTONES, AND EQUITY CONSIDERATION |
| 4.1. | Licensee’s Obligation to Pay Fees, Royalties and Other Payments. As partial consideration for the rights granted by JHU under this AGREEMENT, LICENSEE shall pay to JHU all ROYALTIES, fees, PAST PATENT COSTS, PATENT COSTS,SUBLICENSE NON-ROYALTY CONSIDERATION, and other payments LICENSED PARTIES are required to pay JHU under this AGREEMENT. SALES, actions, or omissions by any LICENSED PARTY are deemed to be SALES, actions, or omissions of LICENSEE. | |
| 4.2. | Upfront License Fee. LICENSEE shall pay to JHU a nonrefundable UPFRONT LICENSE FEE as specified in Exhibit A-2 within thirty (30) days of the EXECUTION DATE. The UPFRONT LICENSE FEE paid by LICENSEE to JHU shall not be credited towards any other payments LICENSEE is required to pay JHU under this AGREEMENT. |
| 4.3. | Annual License Fee. LICENSEE shall pay to JHU annually on or before January 1 of each calendar year the ANNUAL LICENSE FEE as specified in Exhibit A-2. | |
| 4.4. | Patent Costs. LICENSEE shall reimburse JHU for all PAST PATENT COSTS specified in Exhibit A-2 according to the time schedule specified in Exhibit A-2. PATENT COSTS will be invoiced to LICENSEE on a rolling basis as processed by JHU or JHU’s patent counsel and are due and payable within thirty (30) days of receipt by LICENSEE. | |
| 4.5. | Minimum Annual Royalty. By January 1 of each calendar year, LICENSEE shall pay JHU the MINIMUM ANNUAL ROYALTY (“MAR”) specified in Exhibit A-2. MAR payments are non-refundable and will be credited against ROYALTIES incurred by LICENSEE for the calendar year in which the MAR was due. No MAR credits will be applied to ROYALTIES incurred in prior or subsequent calendar years. | |
| 4.6. | Royalties on Licensed Products and Reports. Within forty-five (45) days of the end of each calendar quarter following FIRST COMMERCIAL SALE, LICENSEE shall pay ROYALTIES in accordance with Exhibit A-2 and submit the electronic Excel Quarterly SALES & ROYALTY Report set forth in Exhibit C. ROYALTIES shall be paid on all SALES, use or manufacture of LICENSED PRODUCTS in the LICENSED TERRITORY by all LICENSED PARTIES. | |
| 4.7. | Milestone Payments. Within thirty (30) days of achieving a MILESTONE, LICENSEE shall pay the related milestone payment to JHU as specified in Exhibit A- 3. |
| 4 |
| 4.8. | Private Offering Purchase Rights. As partial consideration in addition to license fees, in the event of any private offering of the LICENSEE’s equity securities for cash (or in satisfaction of debt issued for cash)(also outline on Exhibit A-5): |
| 4.8.1. | JHU and/or its Assignee (as defined below) may purchase for cash up to one percent (1%) of the securities or interests issued in such offering. |
| 4.8.1.1. | “Assignee” means: (a) any entity to which JHU’s participation rights under this Section 1 have been assigned either by JHU or another entity; or (b) any entity that is controlled by JHU. |
| 4.8.2. | In any private offering subject to this AGREEMENT (“Offering”), JHU and/or its Assignee’s purchase right shall be at the same price and on the same terms as the most favored other investors, except that JHU and/or its Assignee shall not have any board representation or board meeting attendance rights. | |
| 4.8.3. | LICENSEE shall give JHU at least thirty (30) days advance written notice of the terms of each Offering, including the names of the investors and the amounts to be invested by each, and JHU may elect to exercise its right of purchase, in whole or in part, by written notice given to the LICENSEE within fifteen (15) business days after receipt of LICENSEE’S notice. To exercise this right, JHU must provide the written notice of its election to invest per the prior sentence and must sign all purchase and shareholder agreements that are signed by the other investors. If JHU and/or its Assignee elects not to purchase or fails to give an election notice within such period, JHU’s purchase right will not apply to the Offering if (and only if and to the extent) it is consummated within ninety (90) days on the same or less favorable (to the investor) terms as stated in LICENSEE’s notice to JHU. |
All rights under this Section 1.4 will not apply to the issuance of stock to employees and other service providers pursuant to a plan approved by LICENSEE’s board of directors, or to shares issued as additional consideration in lending or leasing transactions. In the event of the closing of a firm commitment underwritten public offering, the rights granted in Section 1.4 will terminate (in addition to any earlier termination pursuant to their terms) immediately before such closing.
| 4.8.4. | JHU’s rights respecting equity of or securities in LICENSEE are cumulative. In the event of a conflict, the provision of this AGREEMENT granting greater interests or purchase rights to JHU will govern. | |
| 4.8.5. | This Section 1 shall survive the termination of this AGREEMENT. |
| 4.9. | Patent Expiration and Royalty Adjustments. |
| 4.9.1. | Expiration of Valid Claims. Upon expiration of all VALID CLAIMS, LICENSEE’s ROYALTY obligation shall be reduced by 50%. |
| 5 |
| 4.9.2. | Royalty Stacking. In the event a LICENSEE pays royalties on one or more third party patents (“OTHER ROYALTIES”) as a requirement to make, use or sell a LICENSED PRODUCT, then the LICENSEE may deduct 50% of the amount paid for such OTHER ROYALTY from the ROYALTIES owed to JHU under this AGREEMENT. At no time, however, may the effective ROYALTY rate applicable to a LICENSED PRODUCT that requires OTHER ROYALTIES be less than 50% of the applicable ROYALTY rate as set forth in Exhibit A-2. No deduction under this Section 4.9.2 shall be made for OTHER ROYALTIES paid to an AFFILIATE, division, or corporation sharing a common business location or any corporate officer with LICENSEE or to any SUBLICENSEE. |
| 4.10. | Royalty Duration. LICENSEE’s obligation to pay ROYALTIES on SALES of each LICENSED PRODUCT shall remain in effect for the longer of (i) 10 years from date of FIRST COMMERCIAL SALE, or (ii) the expiration of all VALID CLAIMS, and thereafter LICENSEE shall no longer be obligated to pay ROYALTIES in connection with the SALES of each LICENSED PRODUCT. | |
| 4.10.1. | International Licensed Products. The duration of the LICENSEE’s obligation to pay ROYALTIES shall be determined on a country-by-country basis from the date of FIRST COMMERCIAL SALE to the date of expiration of all VALID CLAIMS. | |
| 4.11. | Sublicense Non-Royalty Consideration. LICENSEE shall pay to JHU the SUBLICENSE NON-ROYALTY CONSIDERATION as stated on Exhibit A-2 within sixty (60) days of receipt of SUBLICENSE NON-ROYALTY CONSIDERATION by LICENSEE. | |
| 4.12. | Assignment Fee. LICENSEE shall pay to JHU an assignment fee as provided for in Exhibit A-4 within sixty (60) days of receipt of assignment consideration from its assignee. | |
| 4.13. | Currency. All payments by LICENSEE to JHU shall be made in U.S. Dollars. Computation of conversion to U.S. Dollars from foreign currency transactions shall be made on a quarterly basis using the exchange rate quoted by United States Federal Reserve Bank for the last business day of the calendar quarter for which payment is due. | |
| 4.14. | Non-U.S. Taxes. LICENSEE shall pay all non-U.S. taxes imposed on all amounts payable by LICENSEE under this AGREEMENT. Such tax payments are not deductible from any payments due to JHU. | |
| 4.15. | Invoicing by JHU. Payments shall be due in accordance with this AGREEMENT, and JHU shall invoice LICENSEE for each payment due. Should JHU send an invoice to LICENSEE, it may do so in electronic form via e-mail sent to the e-mail address supplied by LICENSEE from time to time, and will be deemed received by LICENSEE upon transmission. | |
| 4.16. | Purchase Orders. If at any time LICENSEE requires a Purchase Order to complete payment to JHU under this AGREEMENT or a new Purchase Order number is issued on an annual basis, LICENSEE shall provide Purchase Order No. with JHU Agreement AXXXXX to JHTVReports@JHU.edu or other email address provided by JHTV. Alternatively, LICENSEE may inform JHU of need for or change in Purchase Order number on the electronic Excel Quarterly Royalty and Sales Report |
| 6 |
| 4.17. | Payment Methods. All payments to JHU shall be made either by check or wire transfer, in accordance with the payment instructions set forth in Exhibit A-2 as may be updated from time to time. | |
| 4.18. | Interest. Payments not received when due shall bear interest at the rate of six percent (6%) per annum (compounded monthly) from the date due until paid in full. |
| 5. | ROYALTY REPORTS AND ACCOUNTING |
| 5.1. | Royalty Reports. Beginning with the FIRST COMMERCIAL SALE of a LICENSED PRODUCT, LICENSEE shall thereafter submit to JHU a Quarterly Sales and Royalty Report thirty (30) days after the end of each calendar quarter (even if there are no sales during that quarter), along with royalty payment under Section 4.6. LICENSEE agrees to submit an electronic Excel royalty report using the electronic royalty report template provided by JHU. This report will be in the form of Exhibit C and will state the number, description, and aggregate SALES of LICENSED PRODUCTS during the completed calendar quarter. All indicated columns shall be populated as they pertain to the completed calendar quarter with adjustments and unusual occurrences documented. | |
| 5.2. | Accounting and Audit Rights. Each LICENSED PARTY shall maintain complete and accurate books and records, for no less than seven years, relating to the rights and obligations under this AGREEMENT and any amounts payable to JHU. Such books and records shall include information sufficient to permit JHU to confirm the accuracy and completeness of any payments and reports delivered to JHU and compliance in all other respects with this AGREEMENT. Upon 14 days’ notice, a LICENSED PARTY shall make such books and records available for inspection by JHU or its designee (provided that such designee has signed a confidentiality agreement with terms consistent with those in Article 6 of this Agreement) during normal business hours, to verify any reports, accuracy and completeness of payments and/or compliance with this AGREEMENT. In the event the inspections shows an underpayment to JHU of 5% or more for any quarter during the period examined, LICENSEE shall bear the full cost of the inspection, which shall be due and payable (along with past due ROYALTY, ROYALTY shortfall and other payment amounts plus interest per Section 4.18 from the date that such payments should have been made to JHU) within thirty (30) days of receiving notice from JHU of the inspection results. JHU may exercise this inspection right not more than annually, unless prior inspections show consistent underpayment of 10% or more (in which case JHU may conduct follow up inspections at its discretion). | |
| 5.3. | Statute of Limitations. Notwithstanding any applicable statute of limitation, LICENSEE agrees that it shall pay JHU for any underpayments revealed by an inspection for a period of seven (7) years prior to the inspection. | |
| 5.4. | Final Royalty Report and Payment. Within ninety (90) days of termination of this AGREEMENT, each LICENSED PARTY shall submit a final written Sales and Royalty Report and pay all outstanding amounts due under this AGREEMENT. |
| 7 |
| 6. | CONFIDENTIAL INFORMATION |
| 6.1. | Term of Confidentiality. During the term of this AGREEMENT and for a period of three (3) years thereafter, the parties agree that all CONFIDENTIAL INFORMATION disclosed by a party shall be maintained in confidence by the receiving party and shall not be disclosed by the receiving party to any third party unless agreed to in writing by the disclosing party or compelled by a court of competent jurisdiction; nor shall any such CONFIDENTIAL INFORMATION be used by the receiving party for any purposes other than those contemplated by this AGREEMENT. | |
| 6.2. | Standard for Confidentiality. Each party shall maintain the security of CONFIDENTIAL INFORMATION it receives from the other party by employing reasonable safeguards that are no less secure than those used to protect its own confidential records. | |
| 6.3. | Permitted Disclosures. These obligations respecting CONFIDENTIAL INFORMATION do not preclude disclosures about this AGREEMENT and amounts paid by LICENSED PARTIES as part of routinely prepared summary documents or financial reports, nor do they impede or impair JHU’s exercise of retained research and publication rights pursuant to Section 2.5, provided that JHU not disclose LICENSEE’s CONFIDENTIAL INFORMATION in such publications. |
| 7. | DISCLAIMERS, LIABILITY LIMITATION |
| 7.1. | DISCLAIMER. JHU MAKES NO WARRANTIES UNDER THIS AGREEMENT. ALL TANGIBLE AND INTANGIBLE MATTER, INTELLECTUAL PROPERTY, TECHNOLOGY, RIGHTS, DATA, KNOW-HOW, AND MATERIALS (“DELIVERABLES”) LICENSED, GRANTED, OR PROVIDED BY JHU ARE “AS IS.” JHU MAKES NO REPRESENTATIONS WARRANTIES OF ANY KIND, EITHER EXPRESSED OR IMPLIED, AS TO ANY MATTER INCLUDING WARRANTY OF FITNESS FOR PARTICULAR PURPOSE, MERCHANTABILITY, USEFULNESS, TITLE, NONINFRINGEMENT, VALIDITY, ENFORCEABILITY, USE, UTILITY, SCOPE, OR SUCCESFUL OPERATION OF DELIVERABLES. | |
| 7.2. | LIMITS OF LIABILITY. NEITHER PARTY SHALL BE LIABLE TO THE OTHER FOR INDIRECT, SPECIAL, OR CONSEQUENTIAL DAMAGES, SUCH AS LOSS OF PROFITS OR INABILITY TO USE DELIVERABLES, HOWEVER ARISING, EVEN IF IT HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES. Under no circumstances shall JHU be liable for damages in excess amounts received by JHU under this AGREEMENT during the 12 months prior to the event giving rise to the claim for damages. |
| 8. | INDEMNITY AND INSURANCE |
| 8.1. | Indemnification. LICENSEE and each applicable LICENSED PARTY (each an “Indemnitor” and collectively “Indemnitors”) shall protect, defend, and indemnify the JHU INDEMNITEES from and against any claims, losses, or damages of third parties (i) allegedly arising from or related in any way to any act or omission of an Indemnitor performing or exercising rights granted under this AGREEMENT, or (ii) allegedly caused by or arising in any way from LICENSED PRODUCTS. Indemnitors shall pay to defend the JHU INDEMNITIES against any claim subject to this Section 8.1 with counsel reasonably acceptable to JHU, and shall pay and/or hold the JHU INDEMNITEES harmless as against any judgments, fees, expenses, or other costs arising from or incidental to any such lawsuit, claim, demand or other action, whether or not any JHU INDEMNITEE is named as a party defendant in any such lawsuit and whether or not the JHU INDEMNITEES are alleged to be negligent or otherwise responsible for any injuries to persons or property. |
| 8 |
| 8.1.1. | Exclusions. The LICENSED PARTY Indemnification obligation as stated herein excludes: (i) claims arising solely from the practice by JHU of its retained rights under Section 2.5 of this AGREEMENT; and (ii) claims arising solely from the negligent use or administration by a JHU INDEMNITEE of a LICENSED PRODUCT (but any related claim of product liability or Indemnitor negligence shall remain subject to Indemnification). |
| 8.1.2. | Notice, Cooperation, and Participation. JHU or a JHU Indemnitee shall provide LICENSEE with prompt notice of any claims subject to indemnification, and will provide reasonable cooperation in the investigation and defense of such claims. JHU shall have the right to participate in the defense of any claim with counsel of its choice and at its own expense. JHU shall have the right to approve any settlement against JHU or that imposes any liability or obligation on JHU, such approval not to be unreasonably withheld. JHU shall not be entitled to indemnification if it concludes any settlement or compromise of a claim without the prior written consent of LICENSEE, which consent shall not be unreasonably withheld, delayed, or conditioned. |
| 8.2. | Insurance. LICENSEE shall, continuing throughout the term of this AGREEMENT and for a period of three years thereafter, obtain and maintain, in full force and effect and at LICENSEE’s sole cost and expense, the insurance coverage as set forth in Exhibit E. LICENSEE shall provide written proof of such insurance coverage to JHU within 30 days of EXECUTION DATE or initial coverage, whichever is later, and each renewal thereof. This AGREEMENT and the licenses granted herein shall immediately and automatically terminate in the event LICENSEE or a LICENSED PARTY (as applicable) fails to obtain the required insurance or if the insurance lapses or is cancelled. |
| 8.3. | Survival. The foregoing indemnification obligations shall survive termination or expiration of this AGREEMENT, and shall not be subject to any limitation of liability set forth in this AGREEMENT. |
| 9. | PATENTS |
| 9.1. | Title and Authority. JHU shall retain and hold title to all patents and patent applications included in the PATENT RIGHTS. JHU retains all decision-making authority with respect to patent filing and prosecution of the PATENT RIGHTS. |
| 9 |
| 9.2. | Domestic Filing and Prosecution. JHU shall have sole control over the selection of counsel, filing, prosecution, maintenance and management of all issued patents and pending and future patent applications in the United States that are subject to this AGREEMENT. JHU, at LICENSEE’s expense, shall have the right to file, prosecute and maintain all patents and patent applications included in the PATENT RIGHTS. JHU shall request its patent counsel to timely copy LICENSEE on all official actions and written correspondence with any patent office and to afford LICENSEE an opportunity to comment on prosecution matters. LICENSEE may elect to abandon its participation in, and rights to, a patent application or issued patent filed in the United States, provided that LICENSEE notifies JHU in writing at least ninety (90) days before any due date for any pending Office Action or matter or any maintenance fee due date in the case of an issued patent. Such election shall not relieve LICENSEE of the obligation to reimburse JHU for PATENT COSTS and PAST PATENT COSTS associated with such application that were incurred before JHU received actual notice of LICENSEE’s abandonment. Thereafter, JHU may file, prosecute, and/or maintain such patent applications or patents at its own expense and for its own benefit and any PATENT RIGHTS granted on such applications or patents shall be excluded from the LICENSED PATENTS. Failure to provide such notification may be considered by JHU to be LICENSEE’s authorization to proceed at LICENSEE’s expense. |
| 9.3. | Foreign Filing and Prosecution. Upon LICENSEE’s written request and at LICENSEE’s expense, JHU will file and prosecute PATENT RIGHTS in one or more foreign jurisdiction. JHU or its designee shall have sole control over the selection of counsel, filing, prosecution, maintenance and management of all foreign issued patents and pending and future patent applications that are subject to this AGREEMENT. Upon written notification to JHU and its patent counsel at least ninety (90) days in advance of any filing, response, or fee deadline, LICENSEE may elect to abandon its participation in, and rights to, a patent application filed in a foreign jurisdiction. Such election shall not relieve LICENSEE of the obligation to reimburse JHU for PATENT COSTS and PAST PATENT COSTS associated with such application that were incurred before JHU received actual notice of LICENSEE’s abandonment. Thereafter, JHU may file, prosecute, and/or maintain such foreign patent applications or patents at its own expense and for its own benefit and any PATENT RIGHTS granted on such applications or patents shall be excluded from the LICENSED PATENTS. |
| 9.4. | Common Interest. All non-public information exchanged between JHU and the LICENSED PARTIES or their respective counsel regarding preparation, filing, prosecution, and maintenance of the PATENT RIGHTS shall be deemed CONFIDENTIAL INFORMATION. In addition, the parties acknowledge and agree that, with respect to such preparation, filing, prosecution and maintenance of the PATENT RIGHTS, the interests of the parties are to obtain the strongest patent protection possible, and as such, are aligned and are legal in nature. The parties agree and acknowledge that they have not waived, and nothing in this AGREEMENT constitutes a waiver of, any legal privilege concerning the PATENT RIGHTS or the CONFIDENTIAL INFORMATION, including privilege under the common interest doctrine and similar or related doctrines. |
| 9.5. | INFRINGEMENT. |
| 9.5.1. | Notification of Infringement by third party. Each party will promptly notify the other in writing in the event it discovers, receives notice of, or otherwise reasonably suspects infringement by a third party. |
| 10 |
| 9.5.2. | Suits for Infringement. LICENSEE shall have the first right, at its own expense, to initiate and prosecute an infringement action against one or more third parties to enforce the LICENSED PATENTS in the FIELD OF USE in the LICENSED TERRITORY, provided that LICENSEE: (i) notifies JHU at least ninety (90) days in advance of any such suit; (ii) does not file said action without the prior written consent of JHU; and (iii) carefully considers the views of JHU and the public interest in making its decision whether or not to file suit. LICENSEE: (i) shall not initiate an infringement action in the absence of a good faith belief in the infringement, validity and enforceability of the asserted claims after reasonable investigation, (ii) shall at all times keep JHU informed as to the status of the action and shall consult with JHU throughout the action; and (iii) shall at all times carefully consider the views of JHU with respect to any infringement action, including, for example, choice of litigation counsel, venue, and litigation strategy. LICENSEE shall pay to JHU 20% of any monetary award, settlement or recovery, net of all reasonable LICENSEE and JHU attorneys’ fees and out-of-pocket costs and expenses paid to third parties by LICENSEE and/or JHU in connection with each suit or settlement. | |
| 9.5.3. | Party Communications. All communications concerning a suit or potential suit against a third party between JHU and LICENSEE shall be treated as CONFIDENTIAL INFORMATION and are agreed to be subject to all available privileges and protections including the joint defense privilege and common interest privilege. Settlement or other voluntary final disposition of the suit may not be concluded without the prior written consent of JHU. JHU shall reasonably cooperate in any such litigation at LICENSEE’s expense. | |
| 9.5.4. | JHU’s Secondary Right to Enforce. LICENSEE understands and agrees that JHU has no obligation to bring suit against third parties for infringement of the LICENSED PATENTS. In the event LICENSEE does not initiate an infringement action within ninety (90) days after its discovery of or receiving notification of alleged infringement, JHU may initiate and prosecute such infringement action in its sole discretion and on its own behalf. LICENSEE shall reasonably cooperate in such litigation at JHU’s request, including as a co-plaintiff, and agrees to provide any evidence, witnesses or other support of litigation as needed at its own expense. Upon initiation of an infringement action by JHU, JHU shall have the sole right to seek resolution of the alleged infringement through litigation, settlement agreement or otherwise. After the ninety day period of discovery/notice has elapsed, LICENSEE shall not be permitted to transfer its rights or sublicense the LICENSED PATENTS or otherwise reach an agreement with any suspected infringer that would impact JHU’s action in any way. Any recovery from JHU’s action shall be for JHU’s sole benefit and account. All communications concerning a suit or potential suit against a third party between JHU and LICENSEE shall be treated as CONFIDENTIAL INFORMATION and are agreed to be subject to all available privileges and protections including the joint defense privilege and common interest privilege. |
| 11 |
| 9.6. | Third Party Invalidity Actions. LICENSEE shall defend at LICENSEE’s expense any declaratory judgment or other action brought by a third party naming LICENSEE and/or JHU as a defendant and alleging invalidity of any of the PATENT RIGHTS unless such action is brought as a counterclaim to a suit against the third party initiated by JHU pursuant to JHU’s secondary right to enforce. JHU may, in its sole discretion and at its own expense, assume control of the defense of any third party action naming JHU as a defendant, in which case LICENSEE shall cooperate fully with JHU in such defense at its own expense. | |
| 9.7. | Waiver of Invalidity Claims. LICENSEE, on behalf of itself, AFFILIATEs, and SUBLICENSEEs, understands and agrees that transfer of LICENSED RIGHTS under this AGREEMENT will confer substantial benefits to them, even in the absence of one of more VALID CLAIMS. Such benefits include “early mover” advantage. In addition, LICENSEE on behalf of itself, AFFILIATEs, and SUBLICENSEEs understands and agrees that the consideration paid for LICENSED RIGHTS reflects the nature and risks of early-stage technology, and the consideration required for a license to later stage technology would be significantly higher. Accordingly, each LICENSED PARTY agrees that it shall not initiate any action or proceeding to invalidate PATENT RIGHTS and hereby waives any rights they may have to do so. |
| 9.8. | Patent Challenges. Notwithstanding the foregoing, if a LICENSED PARTY initiates an action or proceeding challenging the validity or scope of PATENT RIGHTS or that a LICENSED PRODUCT practices the PATENT RIGHTS, the following shall apply: |
| (a) | JHU may terminate this AGREEMENT upon written notice to LICENSEE and/or the LICENSED PARTY. | |
| (b) | No payments or reports required by this AGREEMENT shall be suspended or delayed during any challenge to PATENT RIGHTS and no such payments shall be subject to refund or recoupment for any reason. | |
| (c) | Not less than ninety (90) days prior to initiating any challenge to a PATENT RIGHTS, the party challenging PATENT RIGHTS (the “Challenging Party”) shall provide written notice of the expected challenge to JHU which shall include a clear statement of the factual and legal basis for the challenge, and an identification of all prior art, documents, products or other matter the Challenging Party believes to provide a basis for such challenge. | |
| (d) | If such action or proceeding determines that at least one claim of the PATENT RIGHTS is a VALID CLAIM or practiced by a LICENSED PRODUCT, LICENSEE and the Challenging Party shall, thereafter, pay to JHU three times all payment amounts which LICENSEE and Challenging Party would otherwise be required to be paid under this AGREEMENT, other than PATENT COSTS. LICENSEE shall not be obligated to pay increased charges if it is not a party to the challenge to PATENT RIGHTS, has not assisted or facilitated the challenge, and has fully cooperated with JHU in the defense of such challenge. |
| 9.9. | Marking. All LICENSED PRODUCTS shall be marked with the number of the applicable licensed patent(s) in accordance with each country’s patent laws. |
| 12 |
| 10. | DISPUTES |
| 10.1. | Governing Law, Jurisdiction and Venue. This AGREEMENT shall be construed, and legal relations between the parties shall be determined, in accordance with the laws of the State of Maryland applicable to contracts executed and wholly to be performed within the State of Maryland without giving effect to the principles of conflicts of laws. Any disputes between the parties to the AGREEMENT shall be brought in the state or federal courts located in Baltimore, Maryland. Both parties hereby waive their right to a jury trial and consent to jurisdiction in such courts with respect to any disputes between them. |
| 10.2. | Resolution. The parties shall attempt in good faith to resolve all disputes through means other than litigation, such as mediation, arbitration, or structured negotiations. Each party agrees that, prior to initiating litigation, it will confer with other party about alternatives to litigation that may enable them to resolve the dispute fairly and efficiently. |
| 11. | TERM AND TERMINATION |
| 11.1. | Term. The term of this AGREEMENT shall commence on the EFFECTIVE DATE and shall continue until the date of expiration of the last to expire patent included within PATENT RIGHTS, or if no patents issue, then for 20 years from the EFFECTIVE DATE. LICENSEE shall not make, use, sell, import, export or offer for sale any LICENSED PRODUCTS after termination (but not expiration) of this AGREEMENT | |
| 11.2. | Licensee Termination for Convenience. LICENSEE may terminate this AGREEMENT upon ninety (90) days’ advance written notice. | |
| 11.3. | JHU Termination for Cause. JHU may terminate this AGREEMENT upon thirty (30) days’ written notice to LICENSEE in the event of any material breach hereof, provided that LICENSEE does not cure such breach prior to expiration of such thirty (30) day period. JHU may terminate this AGREEMENT immediately upon written notice to LICENSEE in the event of a material breach that is incapable of cure. A material breach may include: |
| (a) | LICENSEE’s delinquency with respect to payment or reporting; | |
| (b) | Failure to timely achieve a MILESTONE specified in Exhibit A-3 or otherwise failing to diligently develop, commercialize, and sell LICENSED PRODUCTS throughout the term of this AGREEMENT; | |
| (c) | Non-compliance with record keeping or audit obligations as stated in Articles 3 and 5 of this AGREEMENT; | |
| (d) | Voluntary bankruptcy or insolvency of LICENSEE. | |
| (e) | Non-compliance with LICENSEE’S insurance obligations. |
| 11.4. | Licensee Obligations Upon Termination or Expiration. Upon expiration or termination of this AGREEMENT for any reason, LICENSEE shall remit payment to JHU for all amounts due or incurred prior to the effective date of termination, and any non-cancellable expenses (such as PATENT COSTS) undertaken prior to termination. | |
| 11.5. | Effect of Termination. Upon termination of this AGREEMENT pursuant to 11.2 or 11.3, all rights and licenses granted by JHU to LICENSEE under this AGREEMENT shall terminate and all rights in, to, and under the LICENSED RIGHTS will revert to JHU. |
| 13 |
| 12. | MISCELLANEOUS |
| 12.1. | Use of Name. LICENSEE may not use the name, trademarks, logos, or trade dress of The Johns Hopkins University, The Johns Hopkins Health System, and any of their constituent parts, such as JHU, Johns Hopkins, Hopkins, the Johns Hopkins Hospital, Johns Hopkins Medicine or any contraction thereof or the name of INNOVATORS in any advertising, promotional literature, Web sites, electronic media applications, sales literature, fundraising documents, or press releases and other print or electronic communications without prior written consent from an authorized representative of JHU. Any request to make use of such names shall be made at least thirty (30) business days’ in advance of any proposed use and may be made by written request through JHTV. JHU shall have the right to list LICENSEE and display the logotype or symbol of LICENSEE on JHU’s website and on JHU publications as a licensee of JHU technology. | |
| 12.2. | Independent Parties. Nothing in this AGREEMENT shall be construed to create any agency, employment, partnership, joint venture or similar relationship between the parties other than that of a licensor/licensee. Neither party shall have any right or authority whatsoever to incur any liability or obligation (express or implied) or otherwise act in any manner in the name or on the behalf of the other, or to make any promise, warranty or representation binding on the other. | |
| 12.3. | Notice of Claim. Each party shall give the other party or its representative prompt notice of any suit or action filed, or of any claim made against them arising out of the performance of this AGREEMENT. | |
| 12.4. | No Assignment. Neither party may assign this AGREEMENT, in whole or in part, without the prior written consent of the other party. Notwithstanding the foregoing, LICENSEE may assign this AGREEMENT in accordance with the terms and transfer fee requirements set forth in Exhibit A-4. | |
| 12.5. | Notices. Any notice under any of the provisions of this AGREEMENT shall be deemed given when deposited in the mail, postage prepaid, registered or certified first class mail or by nationally-recognized private mail carrier and addressed to the applicable party at the address stated below, or such other address as such party shall specify for itself by like notice to other party. Transmission of notice by electronic mail is insufficient to meet the requirements of this provision. |
If to JHU:
Executive Director
Johns Hopkins Technology Ventures
1812 Ashland Avenue, Suite 110
Baltimore, Maryland 21205
If to LICENSEE:
BullFrog AI Holdings, Inc.
325 Ellington Blvd., #317,
Gaithersburg, MD 20878
| 14 |
LICENSEE contacts by agreement function:
| Legal: Vin Singh, MS, MBA | ||
| vin@bullfrogai.com | ||
| Patent: Alan Alfano, PhD | ||
| alan.a@bullfrogai.com | ||
| Licensing: Alan Alfano, PhD | ||
| alan.a@bullfrogai.com | ||
| Billing: Vin Singh, MS, MBA | ||
| vin@bullfrogai.com | ||
| Insurance: Vin Singh, MS, MBA | ||
| vin@bullfrogai.com | ||
| Reporting: Vin Singh, MS, MBA | ||
| vin@bullfrogai.com | ||
| 12.6. | Export Control. Certain of the LICENSED RIGHTS may be subject to United States laws and regulations (including the Arms Export Control Act, as amended, and the Export Administration Act of 1979) controlling the export of technical data, computer software, laboratory prototypes, and other commodities. The transfer of certain technical data and commodities may require a license from the cognizant agency of the United States Government and/or written assurances that such transfers shall not be made to certain foreign countries without prior approval of such agency. LICENSEE or the applicable LICENSED PARTY shall fully comply with such export control laws. JHU makes no representation respecting the requirements for such a license, or that, if required, that such a license will be issued. | |
| 12.7. | Successors and Assigns. This AGREEMENT shall bind and inure to the benefit of the successors and permitted assigns of the parties. | |
| 12.8. | No Waivers; Severability. No waiver of any breach of any provision of this AGREEMENT shall constitute a waiver of any other breach of the same or other provision of this AGREEMENT, and no waiver shall be effective unless made in writing and signed by the party waiving. Any provision of this AGREEMENT prohibited by or unenforceable under any applicable law of any jurisdiction shall as to such jurisdiction be deemed ineffective and deleted without affecting any other provision of this AGREEMENT, which shall be interpreted so as to most fully achieve the intentions of the parties. | |
| 12.9. | Entire Agreement. This AGREEMENT supersedes all previous agreements and understandings relating to its subject matter, whether oral or in a writing, and constitutes the entire agreement of the parties and shall not be amended or altered in any respect except in a writing executed by the parties. | |
| 12.10. | No Agency. LICENSEE agrees that no representation or statement by any JHU employee shall be deemed to be a statement or representation by JHU, and that LICENSEE was not induced to enter this AGREEMENT based upon any statement or representation of JHU, or any employee of JHU. JHU is not responsible for any publications, experiments or results reported by any JHU employee prior to, or after, the EFFECTIVE DATE, including those reported by any of the INNOVATORS. |
| 15 |
| 12.11. | Binding Agreement. Exchange of this AGREEMENT in draft or final form between the parties shall not be considered a binding offer, and this AGREEMENT shall not be deemed final or binding on either party until the final AGREEMENT has been signed by both parties. | |
| 12.12. | Delays or Omissions. Except as expressly provided by this AGREEMENT, no delay or omission to exercise any right, power or remedy accruing to any party, shall impair any such right, power or remedy to such party nor shall it be construed to be a waiver of any such breach or default, or an acquiescence therein, or in any similar breach or default be deemed a waiver of any other prior or subsequent breach or default. Any waiver, permit, consent or approval of any kind or character on the part of any party of any breach or default under this AGREEMENT, or any waiver on the part of any party of any provisions or conditions of this AGREEMENT, must be in writing and shall be effective only to the extent specifically set forth in such writing. All remedies either under this AGREEMENT or by law or otherwise afforded to any party, shall be cumulative and not alternative. | |
| 12.13. | Survival. All representations, warranties, covenants and agreements made in this AGREEMENT and which by their express terms or by implication are to be performed or continue to apply after the execution and/or termination of this AGREEMENT or are prospective in nature shall survive such expiration and/or termination. In addition and for avoidance of doubt, the following articles shall survive any termination or expiration: Articles 5, 6, 7, 8, 9, 10, and 11. | |
| 12.14. | No Third-Party Beneficiaries. Nothing in this AGREEMENT shall be construed as giving any person, firm, corporation or other entity, other than the parties and their successors and permitted assigns, any right, remedy or claim under or in respect of this AGREEMENT or any provision hereof. | |
| 12.15. | Headings. Article headings are for convenient reference and are not a part of this AGREEMENT. All referenced Exhibits are part of this AGREEMENT. | |
| 12.16. | Electronic Signature. Any signature, including any electronic symbol or process affirmatively attached to or associated with this AGREEMENT and adopted by JHU or LICENSEE to sign, authenticate, or accept such contract or record acceptance of the AGREEMENT, hereto shall have the same legal validity and enforceability as a manually executed signature or use of a paper-based recordkeeping system to the fullest extent permitted by applicable law, including the Federal Electronic Signatures in Global and National Commerce Act or any state law based on the Uniform Electronic Transactions Act, and the parties hereby waive any objection to the contrary. |
[The Balance of This Page Intentionally Left Blank - Signature Page Follows]
| 16 |
IN WITNESS WHEREOF, the parties have caused this AGREEMENT to be executed in duplicate counterparts, each of which shall be deemed to constitute an original, effective as of EFFECTIVE DATE. The undersigned verify that they have the authority to bind to this AGREEMENT the party on behalf of which they are executing.
This AGREEMENT includes the following Exhibits:
Exhibit A: Financial Terms
Exhibit A-1: LICENSED RIGHTS, FIELD OF USE, and LICENSED TERRITORY
Exhibit A-2: PATENT COSTS, Fees, ROYALTIES, and Payment Terms
Exhibit A-3: MILESTONES
Exhibit A-4: Permitted Assignment
Exhibit A-5: Equity Consideration – Private Offering Purchase Rights
Exhibit A-6: Table of LICENSED PATENTS
Exhibit A-7: Publication List for LICENSED DATA and LICENSED KNOW-HOW
Exhibit B: Definition of Terms
Exhibit C. Quarterly Sales & Royalty Report Form
Exhibit D: Diligence and Annual Report Form
Exhibit E: Insurance
Johns Hopkins University |
BullFrog AI Holdings, Inc. | ||
| By: |  |
By: |  |
| Name: | Steven L. Kousouris | Name: | Vin Singh, MS, MBA |
| Title: | Executive Director, JHTV | Title: | Founder & CEO |
| Date: | February 22, 2022 | 2:22 PM EST | Date: | February 22, 2022 | 8:22 AM PST |
 |
 |
 |
 |
| 17 |
Exhibit A (A-1, A-2, A-3, A-4, A-5, A-6, A-7)
Exhibit A-1: LICENSED RIGHTS, FIELD OF USE, and LICENSED TERRITORY
|
JHU TECH ID, CASE TITLE, and INVENTORS |
JHU TECH ID #C13270 – “An Improved Formulation of Mebendazole and Drug Combination for Improved Cancer Therapy”
INVENTORS: Gregory Riggins, Avadhut Joshi, Renyuan Bai, Tara Williamson, Verena Staedtke |
| LICENSED PATENTS |
SEE PATENT TABLE BELOW (EXHIBIT A-6) |
LICENSED KNOW-HOW |
SEE
REFERENCE LIST BELOW FOR BOTH KNOW-HOW AND DATA (EXHIBIT A-7) |
| LICENSED DATA | SEE REFERENCE LIST BELOW FOR BOTH KNOW-HOW AND DATA (EXHIBIT A-7) |
| FIELD OF USE | Treatment of any human cancer or neoplastic disease, including in clinical trials |
| LICENSED TERRITORY | WORLDWIDE |
| 18 |
Exhibit A-2
PATENT COSTS, Fees, ROYALTIES, and Payment Terms
| UPFRONT LICENSE FEE | Two hundred fifty thousand US Dollars (USD $250,000), payable as listed below: | |
| (a) | Fifty (50) thousand US Dollars (USD $50,000) upon execution of this AGREEMENT, in partial consideration (due within 30 days of the EFFECTIVE DATE); and | |
| (b) | Two hundred (200) thousand US Dollars (USD $200,000) upon the earlier of (i) completion of BullFrog AI IPO, (ii) $10 million USD financing, or (iii) within nine (9) months of the EFFECTIVE DATE. | |
| PAST PATENT COSTS | $116,903.53 payable as follows: | |
| (a) | $41,561.53 within thirty (30) days of invoice after the Effective Date | |
| (b) | $37,671.00 within four (4) months of the Effective Date | |
| (c) | $37,671.00 within ten (10) months of the Effective Date | |
| MINIMUM ANNUAL ROYALTY (“MAR”) | Due by January 1 of each calendar year:
| |
1st year: USD $5,000 due 1/1/2023 2nd year: USD $10,000 due 1/1/2024 3rd year: USD $20,000 due 1/1/2025 4th year: USD $30,000 due 1/1/2026 5th year, etc.: USD $50,000 due 1/1/2027 (until FIRST COMMERCIAL SALE)
Upon FIRST COMMERCIAL SALE of a LICENSED PRODUCT the MAR shall be: $250,000 due on January 1 in the year after the FIRST COMMERCIAL SALE. | ||
| ROYALTY | 3.5% on LICENSED PRODUCTS | |
| SUBLICENSE NON-ROYALTY CONSIDERATION | The percent of all consideration, as outlined below, received by LICENSEE from a SUBLICENSEE in exchange for grant of SUBLICENSE rights under this AGREEMENT, but excluding (i) any consideration received by LICENSEE for ROYALTIES on SUBLICENSEE SALES (ROYALTIES on SALES by SUBLICENSEES will be treated as if LICENSEE made the SALE), and (ii) any payment of PAST PATENT COSTS or PATENT COSTS made by SUBLICENSEE to LICENSEE. | |
| a) | 20% - Prior to dosing of the first patient in the first Phase II Clinical Trial | |
| b) | 15% - After dosing of the first patient in the first Phase II Clinical Trial, but prior to dosing of a first patient in a Phase III or REGISTRATIONAL CLINICAL TRIAL | |
| c) | 10% after dosing of the first patient in a Phase III or REGISTRATIONAL CLINICAL TRIAL | |
| 19 |
Payment Instructions
Checks are to be made payable to the “Johns Hopkins University.”
All check payments from LICENSEE to JHU shall be sent to:
Executive Director
Johns Hopkins Technology Ventures The Johns Hopkins University
1812 Ashland Avenue, Suite 110
Baltimore, MD 21205
Attention: JHU AGREEMENT No. A40219
or such other addresses which JHU may designate in writing from time to time. .
Wire transfers may be made through:
DOMESTIC ACH & WIRE
Johns Hopkins University – JHTV M&T Bank
1 M&T Plaza
Buffalo, NY 14203
ABA #022000046
Account number: 9864226981 Type of account: depository
CTX format is preferred; CCD+ is also accepted
Attention: JHU AGREEMENT A40219
INTERNATIONAL FED WIRE
Johns Hopkins University – JHTV M&T Bank
1 M&T Plaza
Buffalo, NY 14203
ABA #022000046
Account number: 9864226981 Type of account: depository
CHIPS ABA number: N/A IBAN number: N/A
Attention: JHU AGREEMENT A40219_
LICENSEE shall be responsible for any and all costs associated with wire transfers.
| 20 |
Exhibit A-3
MILESTONES
All milestone payment fees are denoted in United States dollars (USD), unless otherwise noted
| Date or Deadline | Description of MILESTONE | Milestone Payment Fee | |||
| Within 6 months of EFFECTIVE DATE | Financing of $10M in LICENSEE | NO FEE | |||
| None | Validation of EU jurisdictions for granted EPO patent | NO FEE | |||
| Within 6 months of EFFECTIVE DATE | Submission of Development Plan to JHU/JHTV | NO FEE | |||
| Within 36 months of the EFFECTIVE DATE | Enrollment of a first patient in a first Phase 2 clinical trial for the first LICENSED PRODUCT |
NO FEE | |||
| None | COMPLETION of the first Phase 2 clinical trial for each LICENSED PRODUCT | $250,000 | |||
| Within 36 months from the COMPLETION of the first Phase 2 study for the first LICENSED PRODUCT | Enrollment of a first patient in the first REGISTRATIONAL CLINICAL TRIAL | NO FEE | |||
| None | COMPLETION of REGISTRATIONAL CLINICAL TRIAL for each LICENSED PRODUCT | $500,000 (if Phase 3); $250,000 (if Phase 2b) | |||
| None | MARKET APPROVAL for each LICENSED PRODUCT – US (FDA) | $750,000 | |||
| None | MARKET APPROVAL for each LICENSED PRODUCT – outside of US | $500,000 | |||
| None | FIRST COMMERCIAL SALE for the first LICENSED PRODUCT | NO FEE | |||
| None | First twenty (20) million dollars (USD $20,000,000) NET SALES REVENUE of a LICENSED PRODUCT in the United States | $1,000,000 | |||
| None | First year cumulative NET SALES REVENUE of a LICENSED PRODUCT exceeds $100M | $2,000,000 | |||
| None | First year cumulative NET SALES REVENUE of a LICENSED PRODUCT exceeds $500M | $10,000,000 | |||
| None | First year cumulative NET SALES REVENUE of a LICENSED PRODUCT exceeds $1000M (i.e., $1B) | $20,000,000 | |||
| None | COMPLETION of each first-in-human trial for any indication other than Glioblastoma | $250,000 |
| a) | Pursuant to Section 3.1 of the AGREEMENT, milestone payment fees set forth in the above table shall be payable within thirty (30) days of each LICENSED PRODUCT achieving such MILESTONE. | |
| b) | In the event that a Phase I, II and/or Phase III clinical trial is conducted at JHU, LICENSEE is excused from making the accrued related milestone payments fees to JHU until the earlier of, a clinical trial milestone is met where such sites include at least two (2) sites other than JHU or the milestone for the receipt of regulatory approval from the FDA (or marketing approval from a foreign equivalent of the FDA) (“MARKET APPROVAL”) is met. Under such circumstance, LICENSEE agrees that it will pay the accrued Phase I, II and/or Phase III milestone payments fees upon completion and when it submits milestone payment fee to JHU for MARKET APPROVAL. Further, in the event that a Phase I and/or Phase II and/or Phase III clinical trial is not required for MARKET APPROVAL, LICENSEE agrees that it will pay the accrued Phase I and/or Phase II and/or Phase III milestone payment fees otherwise due under Phase I through Phase III milestones when it submits milestone payment fee to JHU for MARKET APPROVAL. | |
| c) | The milestones described above shall be deemed achieved if the objectives are met by LICENSEE or any SUBLICENSEE. Any amounts payable by LICENSEE hereunder may be assigned to, and payable by, a SUBLICENSEE. |
| 21 |
Exhibit A-4
Permitted Assignment
| 1. | LICENSEE may assign this AGREEMENT as part of a sale or merger of substantially all of LICENSEE’s business or assets, regardless of whether such a sale occurs through an asset sale, stock sale, merger or other combination, provided: |
| (a) | LICENSEE provides written notice to JHU at least thirty (30) days in advance of such assignment; | |
| (b) | The assignee agrees, in a writing delivered to JHU, to be bound by all provisions of this AGREEMENT; and | |
| (c) | LICENSEE remits an assignment fee to JHU equal to |
| i. | the greater of twice the MAR applicable to the year when the assignment will be completed; OR | |
| ii. | one hundred thousand US dollars (USD $100,000) |
| 22 |
Exhibit A-5
Equity Consideration – Private Offering Purchase Rights
Private Offering Purchase Rights. In the event of any private offering of the LICENSEE’s equity securities for cash (or in satisfaction of debt issued for cash):
| 1. | JHU and/or its Assignee (as defined below) may purchase for cash up to 1% of the securities or interests issued in such offering. |
| 1.1. | “Assignee” means: (a) any entity to which JHU’s participation rights under this Section 1 have been assigned either by JHU or another entity; or (b) any entity that is controlled by JHU |
| 2. | In any private offering subject to this AGREEMENT (“Offering”), JHU and/or its Assignee’s purchase right shall be at the same price and on the same terms as the most favored other investors, except that JHU and/or its Assignee shall not have any board representation or board meeting attendance rights. |
| 3. | Licensee shall give JHU at least thirty (30) days advance notice of the terms of each Offering, including the names of the investors and the amounts to be invested by each, and JHU may elect to exercise its right of purchase, in whole or in part, by written notice given to LICENSEE within fifteen (15) business days after receipt of LICENSEE’s notice. To exercise this right, JHU must provide the written notice of its election to invest per the prior sentence and must sign all purchase and shareholder agreements that are signed by the other investors. If JHU and/or its Assignee elects not to purchase or fails to give an election notice within such period, JHU’s purchase right will not apply to the Offering if (and only if and to the extent) it is consummated within ninety (90) days on the same or less favorable (to the investor) terms as stated in LICENSEE’s notice to JHU. |
All rights under this Section 1.4 will not apply to the issuance of stock to employees and other service providers pursuant to a plan approved by LICENSEE’s board of directors, or to shares issued as additional consideration in lending or leasing transactions. In the event of the closing of a firm commitment underwritten public offering, the rights granted in Section 1.4 will terminate (in addition to any earlier termination pursuant to their terms) immediately before such closing.
| 4. | JHU’s rights respecting equity of or securities in LICENSEE are cumulative. In the event of a conflict, the provision of this AGREEMENT granting greater interests or purchase rights to JHU will govern. |
This Section 1 shall survive the termination of this AGREEMENT.
| 23 |
Exhibit A-6
Table of LICENSED PATENTS
| JHU ID | Title | Serial Number | File Date | Application Type | Country | Status | Patent Number | Expiration Date | Composition, MoU | |||||||||
| P13270- 01 | An Improved Formulation of Mebendazole and Drug Combination to Improve Anti- cancer Activity |
62/112,706 |
06 Feb 2015 |
Provisional |
US |
Expired |
||||||||||||
| P13270- 02 | An Improved Formulation of Mebendazole and Drug Combination to Improve Anti- cancer Activity | PCT/US2016/016968 | 08 Feb 2016 | PCT | PCT - Parent | Expired | 11 Aug 2016 | Both | ||||||||||
| P13270- 03 | MEBENDAZOLE POLYMORPH FOR TREATEMENT AND PREVENTION OF TUMORS |
15/548,959 |
04 Aug 2017 |
PCT |
US |
GRANTED |
11,110,079 |
08 Feb 2036 |
Both | |||||||||
| P13270- 04 | Mebendazole Polymorph For Treatment And Prevention Of Tumors |
16747414.7 |
08 Feb 2016 |
PCT |
EPO |
GRANTED |
Pending |
08 Feb 2036 |
Both | |||||||||
| P13270- 05 | MEBENDAZOLE POLYMORPH FOR TREATMENT AND PREVENTION OF TUMORS |
253854 |
08 Feb 2016 |
PCT |
Israel |
GRANTED |
253854 |
08 Feb 2036 |
Both | |||||||||
| P13270- | An Improved Formulation of | |||||||||||||||||
| 06 | Mebendazole and Drug Combination to Improve Anti- |
2016800144274 | 08 Feb 2016 |
PCT | China | GRANTED | 1ZL20168- 0014427.4 |
08 Feb 2036 |
Both | |||||||||
| cancer Activity | ||||||||||||||||||
| P13270- | An Improved Formulation of | |||||||||||||||||
| 07 | Mebendazole and Drug Combination to Improve Anti- |
201717028684 | 08 Feb 2016 |
PCT | India | GRANTED | 352734 | 08 Feb 2036 |
Both | |||||||||
| cancer Activity | ||||||||||||||||||
| P13270- 08 | Mebendazole Polymorph For Treatment And Prevention Of Tumors |
2017-541687 |
08 Feb 2016 |
PCT |
Japan |
GRANTED |
6796586 |
08 Feb 2036 |
Both | |||||||||
| P13270- 09 | CONTINUATION: Mebendazole Polymorph For Treatment And Prevention Of Tumors |
17/402,131 |
13 Aug 2021 |
CON |
United States |
PENDING |
Both |
| 24 |
Exhibit A-7
Publication List for LICENSED DATA and LICENSED KNOW-HOW
| 1. | Gallia GL, Holdhoff M, et al. Mebendazole and temozolomide in patients with newly diagnosed high-grade gliomas: results of a phase 1 clinical trial. Neuro-Oncology Advances. 2021;3(1):1-8 |
| 2. | Williamson T, Mendes TB, et al. Mebendazole inhibits tumor growth and prevents lung metastasis in models of advanced thyroid cancer. Endocrine-Related Cancer. 2020:27;123-136 |
| 3. | Skibinski CG, Williamson T, Riggins GJ. Mebendazole and radiation in combination increase survival through anticancer mechanisms in an intracranial rodent model of malignant meningioma. J. Neuro-Oncology. 2018:140;529-538 |
| 4. | Bai RY, Staedtke V, et al. Brain Penetration and Efficacy of Different Mebendazole Polymorphs in a Mouse Brain Tumor Model. Clin Cancer Res. 2015:21(15);3462-3470 |
| 5. | Bai RY, Staedtke V, et al. Antiparasitic mebendazole shows survival benefit in 2 preclinical models of glioblastoma multiforme. Neuro-Oncology. 2011:13(9);974-982 |
| 6. | Bai RY, Staedtke V, et al. Effective treatment of diverse medulloblastoma models with mebendazole and its impact on tumor angiogenesis. Neuro-Oncology. 2014:0;1-10 |
| 7. | Williamson T, Bai RY, et al. Mebendazole and a non-steroidal anti-inflammatory combine to reduce tumor initiation in a colon cancer preclinical model. Oncotarget. 2016:7(42);68571- 68584 |
| 25 |
Exhibit B
DEFINITIONS
“AFFILIATE” means any corporation, licensee, partnership, joint venture or other entity, which controls, is controlled by or is under common control with LICENSEE, as evidenced by the direct or indirect ownership of at least 50% of voting rights governing the entity or the contractual power to control such rights.
“COMBINATION PRODUCT” means a collection or group of products sold together (such as in a kit or package) that contains (i) a LICENSED PRODUCT and (ii) one or more other functional products (“Other Products”) that has been sold separately for use without the LICENSED PRODUCT and which is not essential to the use or practice of the LICENSED PRODUCT. For example, a diagnostic panel comprising a LICENSED PRODUCT and an independent diagnostic biomarker.
“COMPLETION” of a clinical trial milestone means first public release of TOP-LINE data.
“CONFIDENTIAL INFORMATION” means information disclosed by a party (the “Disclosing Party”) to the other party (the “Receiving Party”) in connection with performance of this AGREEMENT that (i) concern the LICENSED RIGHTS and has been maintained by the Disclosing Party as nonpublic or proprietary information, and (ii) is marked Confidential or otherwise expressly designated as Confidential. To be deemed CONFIDENTIAL INFORMATION, oral disclosures must (i) concern the LICENSED RIGHTS, have been maintained by the Disclosing Party as nonpublic or proprietary information, and be described in writing as confidential by the Disclosing Party within fourteen (14) days of disclosure to the Receiving Party. CONFIDENTIAL INFORMATION does not include information that (a) was already in the Receiving Party’s possession before the disclosure by the Disclosing Party; (b) has been published or is later published, unless such publication is a breach of this AGREEMENT; (c) is received by the Receiving Party from a third party not under an obligation of confidentiality; or (d) is independently developed by the Receiving Party’s employees who did not have access to CONFIDENTIAL INFORMATION.
“DISCOVERED PRODUCT” means a product, material, or service that is identified, selected or determined to have utility in whole or in part by the use of a LICENSED PRODUCT, including the use of a screening method or assay covered by the LICENSED RIGHTS.
“EXECUTION DATE” means the date that the last party to sign executes this AGREEMENT. “FIELD OF USE” is defined in Exhibit A-1.
“FIRST COMMERCIAL SALE” means the first transfer by a LICENSEE to a third party for value of a LICENSED PRODUCT, with the exemption of materials transferred for use in a clinical trial at a nominal cost to the recipient.
| 26 |
“HUMANITARIAN PURPOSE” means practice of LICENSED RIGHTS in the prevention or treatment of disease in humans by or on behalf of any QUALIFIED HUMANITARIAN ORGANIZATION (including, for clarity, practice of LICENSED RIGHTS by contractors, manufactures or distributors acting for or on behalf of such QUALIFIED HUMANITARIAN ORGANIZATIONs on a fee-for-service, fee-for-product or charitable basis): (i) to manufacture LICENSED PRODUCTS anywhere in the world for the sole and express purposes of distribution and use of such LICENSED PRODUCTS in one or more LEAST DEVELOPED COUNTRIES, and (ii) to sell or otherwise distribute LICENSED PRODUCTS for use solely in one or more LEAST DEVELOPED COUNTRIES; provided, however, that sales and distribution of LICENSED PRODUCTS shall not be deemed made for humanitarian purposes unless products are distributed at locally-affordable prices.
“INNOVATORS” means the individuals who invented, authored, or created the LICENSED RIGHTS as identified in in Exhibit A-1.
“JHU INDEMNITEES” means JHU, The Johns Hopkins Hospital, The Johns Hopkins Health System Corporation, and their affiliated entities, their present and former trustees, officers, INNOVATORS, agents, faculty, employees and students.
“LEAST DEVELOPED COUNTRY” means those jurisdictions so defined by the United Nations Country Classification in the most recent United Nations’ publication “Statistical Annex.”
“LICENSED DATA” means the data specified in Exhibit A-1 that exists as of the EFFECTIVE DATE of this AGREEMENT.
“LICENSED KNOW-HOW” means the know-how described in Exhibit A-1 that exists as of the EFFECTIVE DATE of this AGREEMENT.
“LICENSED PARTIES” means LICENSEE, AFFILIATE, and/or SUBLICENSEE (as applicable).
“LICENSED PATENTS” means the patents and patent applications listed on Exhibit A-1, and includes any foreign patent applications sharing the same disclosure, and any divisional, continuation, or reexamination applications of the listed patents or applications, and every patent that issues or reissues from such applications.
“LICENSED PRODUCT” means any service, process, method, material, compositions, drug, or other product that (i) comprises, constitutes, or embodies the LICENSED RIGHTS, (ii) requires use or practice of the LICENSED RIGHTS by LICENSED PARTIES or their customers, or (iii) is a DISCOVERED PRODUCT.
“LICENSED RIGHTS” means all rights respecting LICENSED PATENTS, LICENSED DATA, and LICENSED KNOW-HOW granted to LICENSEE in Article 2 of this AGREEMENT.
“LICENSED TERRITORY” means the territory specified in Exhibit A.-1. “MILESTONE” means a diligence milestone or event specified in Exhibit A-3.
“NET SALES REVENUE” means and includes the gross value of everything of value received by LICENSED PARTIES as consideration for the SALE of LICENSED PRODUCTS or COMBINATION PRODUCTS, including the fair market value of equity, intangible rights, services and other things of value directly provided in return for SALES except for SUBLICENSEE NON- ROYALTY CONSIDERATION, as that term is defined in Exhibit A-2 of this AGREEMENT.
NET SALES REVENUE generated from COMBINATION PRODUCTS shall be determined with the formula: COMBINATION PRODUCT NET SALES REVENUE = NET SALES REVENUE*C/(C+D), where C is the total gross invoice price of the LICENSED PRODUCT when sold separately and D is the total gross invoice price of the Other Product(s) when sold separately. In the event that no such separate sales are made of the LICENSED PRODUCT or Other Product in such COMBINATION PRODUCT during the royalty paying period in question, NET SALES REVENUE, for the purposes of determining ROYALTY payments shall be calculated using the above formula where C is the commercial value of the LICENSED PRODUCT sold separately and D is the commercial value of the Other Product(s) active ingredients or components sold separately. Any such estimates shall be determined using criteria to be mutually agreed upon by the parties. Such estimates shall be reported to JHU with the reports to be provided pursuant to Section 5.1 hereof. The term “Other Products” does not include solvent, diluents, excipients, buffers or the like used in formulating a product.
| 27 |
NET SALES REVENUE excludes the following items, provided they are separately invoiced to and paid by a purchaser of LICENSED PRODUCTS and thereafter paid or remitted by a LICENSED PARTY:
| ● | import, export, excise and sales taxes, and custom duties; | |
| ● | shipping charges and transportation from the place of manufacture to the customer’s premises or point of installation; | |
| ● | cash, trade or quantity discounts actually granted to end users; | |
| ● | patient assistance and co-pay programs; | |
| ● | sales rebates actually paid or credited to end users including managed care rebates and chargebacks; and | |
| ● | allowances or credits to end users because of rejections or returns. |
For clarity, NET SALES REVENUE shall not include SALES between LICENSED PARTY and its AFFILIATES or SUBLICENSES for the purpose of subsequent resale to a third party, but shall include SALES by AFFILIATES or SUBLICENSEES to Third Parties. In the event that the LICENSED PRODUCT is consumed, depleted or exhausted by the AFFILIATE or SUBLICENSEE, then such use shall be deemed a SALE.
“PATENT COSTS” means all costs of prosecuting and maintaining any LICENSED PATENT, including reasonable attorneys’ fees and expenses, and fees for patent filing(s), maintenance, annuities, translation, and defense against claims of infringement or invalidity, including fees and costs incurred in administrative proceedings or disputes pursuant to the America Invents Act of 2011 (such as an Inter Partes Review, Post Grant Review or Derivation Proceedings before the U.S. Patent Trial and Appeal Board), incurred by JHU. PATENT COSTS excludes PAST PATENT COSTS.
“PAST PATENT COSTS” means all PATENT COSTS that are incurred by JHU prior to the EXECUTION DATE of this AGREEMENT and are able to be billed to LICENSEE on the EXECUTION DATE. For the avoidance of doubt, those PATENT COSTS incurred before the EXECUTION DATE but not available for billing until after the EXECUTION DATE will be billed as PATENT COSTS.
“PATENT RIGHTS” means the rights granted to LICENSEE in respect of the LICENSED PATENTS (and subject to the rights reserved or maintained by JHU).
| 28 |
“QUALIFIED HUMANITARIAN ORGANIZATION” means any governmental agency, non- governmental agency or other not-for-profit organization that has as one of its bona fide missions to address the public health needs of underserved populations on a not-for-profit basis. For clarity, QUALIFIED HUMANITARIAN ORGANIZATIONS do not include non-governmental agencies and not-for-profit organizations that are formed or established for the benefit of any for-profit entity.
“REGISTRATIONAL CLINICAL TRIAL” means, with respect to a given Product, either: (a) a Phase III Clinical Trial with such Product; or (b) a Phase IIb Clinical Trial that, at the time of commencement, is expected to be the basis for initial Regulatory Approval of such Product.
“ROYALTIES” means payments owed to JHU in consideration of the rights granted to LICENSED PARTIES under this AGREEMENT that are determined as a percentage of NET SALES REVENUE as explicitly set forth in Exhibit A-2 of this AGREEMENT.
“SALE” means a sale, license, lease, performance, transfer, delivery, contract to provide, or other disposition or conveyance for value of a LICENSED PRODUCT.
“SUBLICENSE” means an agreement in which LICENSEE (i) grants or otherwise transfers any of the LICENSED RIGHTS, (ii) agrees not to assert or seek a legal remedy for the practice of LICENSED RIGHTS, or (iii) creates an obligation to grant, assign or transfer any LICENSED RIGHTS to any other entity (other than an AFFILIATE).
“SUBLICENSEE” means any person or entity to which LICENSEE has granted a SUBLICENSE under this AGREEMENT.
“SUBLICENSE NON-ROYALTY CONSIDERATION” is defined in Exhibit A-2 of this AGREEMENT.
“TOP-LINE DATA” means, with respect to a clinical study, a summary of patient demographic data, data for the primary endpoint, and safety data derived from the unblinded, locked clinical trial database.
“VALID CLAIM” means (i) any claim of an issued and unexpired patent within the LICENSED PATENTS that has not been (a) conclusively revoked or held unenforceable, unpatentable or invalid by a competent court or tribunal and which is unappealable or unappealed in the time allowed for appeal, and (b) irrevocably disclaimed, cancelled, withdrawn or abandoned or admitted to be invalid or unenforceable through reissue, disclaimer or otherwise; or (ii) a pending claim of a pending patent application within the LICENSED PATENTS.
| 29 |
Exhibit C
Quarterly Sales and Royalty Report
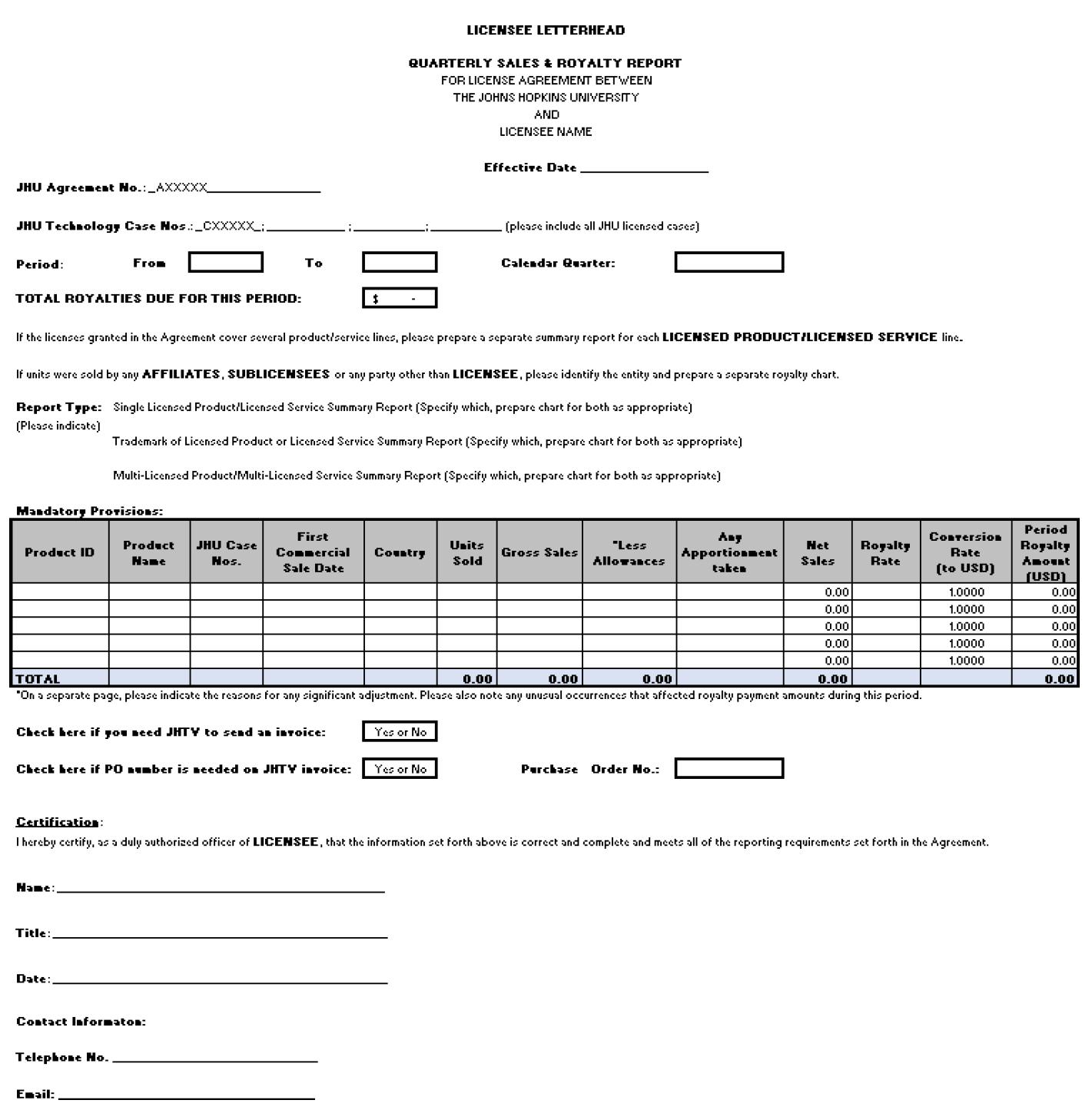
| 30 |
Exhibit D
Diligence and Annual Report
LICENSEE Name: _______________________________________________________________
JHU Agreement Number: ___ A40219 __________________________________________________
JHU Reference Number(s) C13270, _________ , __________ , __________ ,
Reporting Period: From ______________ To _______________
A description of progress by LICENSED PARTIES toward commercialization of LICENSED PRODUCTS, including work completed, key scientific discoveries, summary of work-in- progress, current schedule of anticipated events or MILESTONES, market plans (if any), significant corporate transactions and documents sufficient to evidence each.
A description and documentation of all FDA or other governmental filings and/or approvals regarding any LICENSED PRODUCT or LICENSED RIGHTS.
Certificate of Insurance or other evidence of insurance
________is attached
Identification of all LICENSED PARTIES (AFFILIATE and SUBLICENSEE):
_________NONE
_________List attached with description of rights exercised.
SUBLICENSE(s) entered during the year:
_________NONE
(copy of each SUBLICENSE attached)
A description of any Material Event (e.g., change of control, name change or other significant change related to this AGREEMENT or LICENSEE:
_________NONE
Details:
| 31 |
SEND DILIGENCE AND ANNUAL DILIGENCE REPORT TO:
| Via mail or private mail carrier: | Via email (Preferred): |
| Licensee Reporting Group | JHTVReports@JHU.EDU |
| Johns Hopkins Technology Ventures | Expect Auto-Reply |
| The Johns Hopkins University | No Auto-Reply? |
| 1812 Ashland Avenue, Suite 110 | Contact: |
| Baltimore, MD 21205 | Marlene Moore at |
| Telephone for overnight courier: 410-614-0300 | mmoore26@jhmi.edu or 410-614-0300 |
Interested in reporting via our Licensee Reporting Portal? Contact us at JHTVReports@JHU.edu to request details about this reporting option.
| 32 |
Exhibit E
Required Insurance Coverages
| 1. | Assumption of Liability. LICENSEE hereby assumes full liability for any and all lawsuits, claims, demands, judgments, costs, fees (including attorney’s fees), expenses, injuries or losses arising from or relating to its use of the LICENSED PRODUCTS. |
| 2. | Insurance. LICENSEE will obtain and maintain Comprehensive General Liability Insurance with a reputable and financially secure insurance carrier acceptable to JHU. Prior to initial human testing or FIRST COMMERCIAL SALE of any LICENSED PRODUCT, LICENSEE will obtain and maintain in addition to the Comprehensive General Liability Insurance, Product Liability Insurance with a reputable and financially secure insurance carrier acceptable to JHU, to cover any liability arising from or relating to the LICENSED PRODUCTS. The insurance policy shall provide minimum coverage in the amounts and subject to the provisions below. |
| 3. | General. LICENSEE shall obtain and maintain, in full force and effect and at LICENSEE’s sole cost and expense insurance policies providing: |
| a) | Commercial general liability insurance (including coverage and any necessary endorsements for products /completed operations as well as for clinical trials if any such trials are to be performed by or on behalf of LICENSEE) which provides, for each annual policy period, coverage of no less than the minimum limits specified below for injury, death and property damage resulting from each occurrence during the policy period; and | |
| b) | If required by law, worker’s compensation insurance. |
| 4. | Initial Policy Limits. The commercial general liability and products liability coverages shall have the following minimum limits: |
| a) | Commercial general liability: one million dollars ($1,000,000) each occurrence, two million dollars ($2,000,000) general aggregate. LICENSEE shall have thirty (30) days following the Effective Date to obtain such coverage. | |
| b) | Products liability: From the date immediately prior to initial human testing or first Commercial SALE: $5,000,000 per claim and $10,000,000 in the aggregate. | |
| c) | JHU may periodically evaluate the adequacy of the minimum coverage of insurance and coverage limits specified in this AGREEMENT. JHU reserves the right to require LICENSEE to adjust the insurance coverage by modifying the types of required coverages, the limits and/or financial rating and/or the method of financial rating of LICENSEE’s insurers as such changes are required of JHU by its insurance carrier. JHU shall provide LICENSEE with reasonable notice, contingent on JHU receiving timely notice from its insurance carrier, of any proposed modification, and, if so requested by LICENSEE, discuss any proposed modifications in good faith. |
| 5. | Policy Requirements. Each policy of insurance required by this AGREEMENT shall: |
| a) | be issued by reputable and financially secure insurance carriers having at least an A- rating (A- rating or above by A.M. Best) and an A.M. Best Class Size of at least VIII, | |
| b) | list each of JHU, its trustees, officers, employees, faculty, staff, students, agents and their successors, heirs and assigns as additional insureds, |
| 33 |
| c) | be endorsed to provide that the insurer waives all subrogation rights it has or may have against any additional insured, and | |
| d) | be primary in respect of all additional insureds. |
| 6. | Evidence of Insurance. LICENSEE shall provide JHU with a Certificate of Insurance from each such insurer which evidences compliance by LICENSEE with its obligations under this AGREEMENT. Upon the request of JHU, LICENSEE shall provide JHU with a copy of the policy, status of claims and claims history respecting any of the insurance required to be maintained by LICENSEE under this AGREEMENT. Further, LICENSEE will not cancel or fail to renew the identified insurance without giving JHU at least thirty (30) days’ prior written notice of such cancellation. |
| 7. | Primary Coverage. All insurance of LICENSEE will be primary coverage; other insurance of JHU and JHU Indemnities will be excess and noncontributory. |
| 8. | Clarifications. For the avoidance of doubt, the minimum insurance coverage and limits set forth in this AGREEMENT do not constitute a limitation on LICENSEE’s liability or obligations to indemnify or defend JHU and the JHU INDEMNITEES and any other additional insured under this AGREEMENT. |
| 34 |