File pursuant to Rule 424(b)(3)
Registration No. 333-276740
1,028,710 shares of Common Stock
And Warrants to Purchase 1,028,710 shares of Common Stock
Pre-Funded Warrants to Purchase up 478,429 shares of Common Stock
and Warrants to Purchase 478,429 shares of Common Stock
Underwriter Warrants to Purchase Up to 90,428 Shares of Common Stock
BULLFROG AI HOLDINGS, INC.
This is a firm commitment offering (“Offering”) of shares of common stock, par value $0.00001 per share (“common stock”) of Bullfrog AI Holdings, Inc. (the “Company”, “we”, “us”, “our”). Of the total shares being offered under this prospectus, the Company is offering 1,028,710 shares of common stock (the “Company Shares”) and accompanying warrants (the “Company Warrants”) to purchase up to 1,028,710 shares of common stock (the “Company Warrant Shares”) at a public offering price of $3.782 per Company Share and accompanying Company Warrant. As part of the compensation, the Company is also issuing 90,428 warrants to the Representative of the underwriters (the “Underwriter’s Warrants,” together with the Company Warrants and Pre-Funded Warrants, “Warrants”) to purchase 90,428 shares of common stock (the “Underwriter Warrant Shares,” together with the Company Warrant Shares and Pre-Funded Warrant Shares, the “Warrant Shares”).
We are also offering 478,429 pre-funded warrants (each a “Pre-Funded Warrant”) to purchase up to 478,429 shares of our common stock, exercisable at an exercise price of $0.001 per share, to those purchasers, whose purchase of Company Shares in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this Offering. The purchase price of each Pre-Funded Warrant and accompanying Company Warrant is $3.781. The Pre-funded Warrants will be immediately exercisable and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full.
We have granted a 45-day option to the representative of the underwriters to purchase up to 226,071 additional Company Shares from and/or 226,071 Pre-funded Warrants and/or 226,071 Company Warrants solely to cover over-allotments, if any.
Our common stock and tradeable warrants are quoted on The Nasdaq Capital Market LLC (“Nasdaq”) under the symbols “BFRG” and “BFRGW,” respectively. We have not applied, and do not intend to apply, to list the Pre-Funded Warrants or the Company Warrants on Nasdaq. On January 31, 2024, the closing price as reported on The Nasdaq Capital Market was $4.18 per share.
We are an emerging growth company under the Jumpstart our Business Startups Act of 2012, or JOBS Act, and, as such, may elect to comply with certain reduced public company reporting requirements for future filings. Investing in our common stock involves a high degree of risk.
Investing in our common stock is highly speculative and involves a high degree of risk. See “Risk Factors” beginning on page 7 of this prospectus for a discussion of information that should be considered in connection with an investment in our securities.
| Per Company Share and Company Warrant | Per Pre-Funded Warrant and Company Warrant | Total Offering
without Exercise of Over-Allotment Option (4) (5) | Total Offering
with Full Exercise of Over-Allotment Option (4) | |||||||||||||
| Public offering price | $ | 3.782 | $ | 3.781 | $ | 5,700,000 | $ | 6,555,000 | ||||||||
| Underwriting discount and commissions (8.0%) (1)(2) | $ | 0.303 | $ | 0.302 | $ | 456,000 | $ | 524,400 | ||||||||
| Proceeds to Company (before expenses)(3) | $ | 3.479 | $ | 3.478 | $ | 5,244,000 | $ | 6,030,600 | ||||||||
| (1) | See “Underwriting” for a description of compensation payable to the underwriters. | |
| (2) | Does not include a non-accountable expense allowance equal to 1% of the public offering price payable to WallachBeth Capital LLC (the “Representative”), the representative of the underwriters or the reimbursement of certain expenses of the underwriters. See “Underwriting” for a description of compensation payable to the underwriters. | |
| (3) | Does not include offering expenses including, without limitation, legal, accounting, auditing, escrow agent, transfer agent, other professional, printing, advertising, travel, marketing, blue-sky compliance and other expenses of this Offering. The total expenses of this Offering, excluding the underwriter’s discount and expenses, will be approximately $254,000. | |
| (4) | Assumes no exercise of the Company Warrants. | |
| (5) | Assumes no Pre-Funded Warrants are sold. |
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the securities to purchasers on or about February 5, 2024.
Sole Book-Running Manager
WALLACHBETH CAPITAL LLC
The date of this prospectus is January 31, 2024
TABLE OF CONTENTS
You should rely only on information contained in this prospectus. We have not, and the underwriters have not, authorized anyone to provide you with additional information or information different from that contained in this prospectus. Neither the delivery of this prospectus nor the sale of our securities means that the information contained in this prospectus is correct after the date of this prospectus. This prospectus is not an offer to sell or the solicitation of an offer to buy our securities in any circumstances under which the offer or solicitation is unlawful or in any state or other jurisdiction where the offer is not permitted.
For investors outside the United States: Neither we nor the underwriters have taken any action that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities covered hereby and the distribution of this prospectus outside of the United States.
The information in this prospectus is accurate only as of the date on the front cover of this prospectus. Our business, financial condition, results of operations and prospects may have changed since those dates.
No person is authorized in connection with this prospectus to give any information or to make any representations about us, the securities offered hereby or any matter discussed in this prospectus, other than the information and representations contained in this prospectus. If any other information or representation is given or made, such information or representation may not be relied upon as having been authorized by us.
Neither we nor the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than the United States. You are required to inform yourself about, and to observe any restrictions relating to, this offering and the distribution of this prospectus.
| i |
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information you should consider before investing in our securities. Before investing in our securities, you should carefully read this entire prospectus, including our consolidated financial statements and the related notes thereto and the information set forth under the sections “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes thereto, in each case included in this prospectus. Some of the statements in this prospectus constitute forward-looking statements. See “Cautionary Note Regarding Forward-Looking Statements.” Except as otherwise indicated, references to “we”, “us”, “our”, and the “Company” refer to Bullfrog AI Holdings, Inc. and its wholly-owned subsidiaries.
Business Overview
Most new therapeutics will fail at some point in preclinical or clinical development. This is the primary driver of the high cost of developing new therapeutics. A major part of the difficulty in developing new therapeutics is efficient integration of complex and highly dimensional data generated at each stage of development to de-risk subsequent stages of the development process. Artificial Intelligence and Machine Learning (AI/ML) has emerged as a digital solution to help address this problem.
We use artificial intelligence and machine learning to advance medicines for both internal and external projects. We are committed to increasing the probability of success and decreasing the time and cost involved in developing therapeutics. Most current AI/ML platforms still fall short in their ability to synthesize disparate, high-dimensional data for actionable insight. Our platform technology, named, bfLEAP™, is an analytical AI/ML platform derived from technology developed at The Johns Hopkins University Applied Physics Laboratory (JHU-APL), which is able to surmount the challenges of scalability and flexibility currently hindering researchers and clinicians by providing a more precise3, multi-dimensional understanding of their data. We are deploying bfLEAP™ for use at several critical stages of development for internal programs and through strategic partnerships and collaborations with the intention of streamlining data analytics in therapeutics development, decreasing the overall development costs by decreasing failure rates for new therapeutics, and impacting the lives of countless patients that may otherwise not receive the therapies they need.
The bfLEAP™ platform utilizes both supervised and unsupervised machine learning - as such, it is able to reveal real/meaningful connections in the data without the need for a prior hypothesis. Supervised machine learning uses labeled input and output data, while an unsupervised learning algorithm does not. In supervised learning, the algorithm “learns” from the training dataset by iteratively making predictions on the data and adjusting for the correct answer. Unsupervised learning, also known as unsupervised machine learning, uses machine learning algorithms to analyze and cluster unlabeled datasets. These algorithms discover hidden patterns or data groupings without the need for human intervention. Algorithms used in the bfLEAP™ platform are designed to handle highly imbalanced data sets to successfully identify combinations of factors that are associated with outcomes of interest.
Together with our strategic partners and collaborators, our primary goal is to improve the odds of success at any stage of pre-clinical and clinical therapeutics development. Our primary business model is improving the success and efficiency of drug development which is accomplished either through acquisition of drugs or partnerships and collaborations with companies that are developing drugs. We hope to accomplish this through strategic acquisitions of current clinical stage and failed drugs for in-house development, or through strategic partnerships with biopharmaceutical industry companies. We are able to pursue our drug asset enhancement business by leveraging a powerful and proven AI/ML platform (trade name: bfLEAP™) initially derived from technology developed at JHU-APL. We believe the bfLEAP™ analytics platform is a potentially disruptive tool for analysis of pre-clinical and/or clinical data sets, such as the robust pre-clinical and clinical trial data sets being generated in translational R&D and clinical trial settings. In November 2021, we amended the agreement with JHU-APL to include additional advanced AI technology. On July 8, 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from JHU-APL for the additional technology developed to enhance the bfLEAP™ platform. The July 8, 2022 JHU-APL license provides the Company with new intellectual property and also encompasses most of the intellectual property from the February 2018 license.
We believe bfLEAP™ will inform/enable decision making throughout the development cycle:
| ● | 1. Discovery Phase - Analyze and categorize discovery phase data to better define highest-value leads from groups of candidates, for advancement to preclinical phase of development. Integrate data from high-throughput screening, pharmacodynamics assays, pharmacokinetics assays, and other key data sets to create the most accurate profile of a pool of therapeutic candidates. There is often a high degree of similarity among closely related therapeutics in a candidate pool - bfLEAP™ is able to harmonize disparate data streams for a more nuanced understanding of each candidate’s characteristics/potency. |
| 3 | In an August 2021 publication in DeepAI.org (https://deepai.org/publication/random-subspace-mixture-models-for-interpretable-anomaly-detection), the algorithms used in bfLEAP were compared to 10 of the most popular clustering algorithms in the world using 12 data sets. The end result showed that the algorithms used in bfLEAP had the highest average score when measuring speed and accuracy of prediction. The bfLEAP platform currently has more advanced versions of these algorithms and is applying them in multiple data analytics projects. |
| 1 |
| ● | 2. Pre-Clinical Data - Large-scale/multivariate analysis of pre-clinical and/or early-stage clinical data sets. In these settings, bfLEAP could be used to find novel drug targets, elucidate mechanism of action (MOA), predict potential off-target effects/side effects, uncover specific genetic/phenotypic background(s) with highest correlation to therapeutic response, etc. These insights from bfLEAP™ analysis can be used to inform decision making/study design at the subsequent step(s) of therapeutic/diagnostic development, including first-inhuman/Phase I RCTs. |
| ● | 3. Clinical Development - Advanced/multivariate analysis of PhI and/or PhII clinical trials data, to find niche populations of highly responsive patients and/or inform patient selection for later-stage CT(s). This can be used to decrease overall study risk for larger clinical trials - including Phase II trials, and any Phase III Registration Clinical Trials. The bfLEAP™ platform analysis can also be used to more precisely understand complex correlations between therapeutic treatment and adverse events, side effects, and other undesirable responses which could jeopardize clinical trial success. |
Our platform is agnostic to the disease indication or treatment modality and therefore we believe that it is of value in the development of biologics or small molecules.
The process for our drug asset enhancement program is to:
| ● | acquire the rights to a drug from a biopharmaceutical industry company or academia; | |
| ● | use the proprietary bfLEAP™ AI/ML platform to determine a multi-factorial profile for a patient that would best respond to the drug; | |
| ● | rapidly conduct a clinical trial to validate the drug’s use for the defined “high-responder” population; and | |
| ● | divest/sell the rescued drug asset with the new information back to a large player in the pharma industry, following positive results of the clinical trial. |
As part of our strategy, we will continue evolving our intellectual property, analytical platform and technologies, build a large portfolio of drug candidates, and implement a model that reduces risk and increases the frequency of cash flow from rescued drugs. This strategy will include strategic partnerships, collaborations, and relationships along the entire drug development value chain, as well as acquisitions of the rights to developing failed drugs and possibly the underlying companies.
To date, we have not conducted clinical trials on any pharmaceutical drugs and our platform has not been used to identify a drug candidate that has received regulatory approval for commercialization. However, we currently have a strategic relationship with a leading rare disease non-profit organization for AI/ML analysis of late stage clinical data. We have also positioned the Company to acquire the rights to a series of preclinical and early clinical drug assets from universities, as well as a strategic collaboration with a world renowned research institution to create a HSV1 viral therapeutic platform to engineer immunotherapies for a variety of diseases. In addition, we have signed exclusive world-wide license agreements with Johns Hopkins University for a cancer drug that targets glioblastoma (brain cancer), pancreatic cancer, and other cancers. We have also signed an exclusive worldwide license with George Washington University for another cancer drug that targets hepatoceullar carcinoma (liver cancer), and other liver diseases.
Our platform was originally developed by the JHU-APL. JHU-APL uses the same technology for applications related to national defense. Over several years, the software and algorithms have been used to identify relationship, patterns, and anomalies, and make predictions that otherwise may not be found. These discoveries and insights provide an advantage when predicting a target of interest, regardless of industry or sector. We have applied the technology to various clinical data sets and have identified novel relationships that may provide new intellectual property, new drug targets, and other valuable information that may help with patient stratification for a clinical trial thereby improving the odds for success. The platform has not yet aided in the development of a drug that has reached commercialization. However, we own one drug candidate that has completed a phase 1 trial and a second candidate that is in the preclinical stages. Our aim is to use our technology on current and future available data to help us better determine the optimal path for development.
While we have not generated significant revenues from our AI/ML operations, we anticipate generating revenue in the future from the following three sources:
Contract Services
Our fee for service partnership offering model is designed for biopharmaceutical companies, as well as other organizations, of all sizes that have challenges analyzing data throughout the drug development process. We provide the customer with an analysis of large complex data sets using our proprietary Artificial Intelligence / Machine Learning platform called bfLEAP™. This platform is designed to predict targets of interest, patterns, relationships, and anomalies. Our service model involves a cash fee plus the potential for rights to new intellectual property generated from the analysis, which can be performed at the discovery, preclinical, or clinical stages of drug development.
Collaborative Arrangements
We plan to enter into collaborative arrangements with biotechnology and pharmaceutical companies who have drugs that are in development or have failed late Phase 2 or Phase 3 trials. The collaborations may also be at the discovery or preclinical stages of drug development. Our revenue will be a combination of fee for service cash payments and success fees based on achieving certain milestones as determined by each specific arrangement. There may also be fees or legal rights associated with the development of new intellectual property.
| 2 |
Acquisition of Rights to Certain Drugs
We may acquire the rights to drugs that have failed late Phase 2 or Phase 3 trials and generate revenues by using our platform to accurately determine the profile of patients that would respond to the drugs, conduct a clinical trial to test our findings either independently or with a clinical partner, and finally sell the drug back to pharmaceutical companies. We have and may continue acquiring the rights to drugs that have not yet failed any trials. We will use our technology to improve the chances for success, conduct a trial, and divest the asset. When divesting assets, the transaction may involve a combination of upfront payments, milestone payments based on clinical success, and royalties on sales of the product.
Our bfLEAP™ Analytics Platform
We are able to pursue our drug rescue business by leveraging a powerful and proven AI/ML platform (trade name: bfLEAP™) derived from technology developed at The Johns Hopkins University Applied Physics Laboratory (JHU-APL). The bfLEAP™ platform is based on an exclusive, world-wide license granted by Johns Hopkins University Applied Physics Laboratory. The license covers three (3) issued patents, as well as a new provisional patent application, non-patent rights to proprietary libraries of algorithms and other trade secrets, which also includes modifications and improvements. On July 8, 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from JHU-APL for the additional technology developed to enhance the bfLEAP™ platform. The new license provides additional intellectual property rights including patents, copyrights and knowhow to be utilized under the Company’s bfLEAP™ analytical AI/ML platform. Under the terms of the new License Agreement, JHU will be entitled to eight (8%) percent of net sales for the services provided by the Company to other parties and 3% for internally development drug projects in which the JHU license was utilized. The new license also contains tiered sub licensing fees that start at 50% and reduce to 25% based on revenues.
We believe the bfLEAP™ analytics platform is a potentially disruptive tool for analysis of pre-clinical and/or clinical data sets, such as the robust pre-clinical and clinical trial data sets being generated in translational R&D and clinical trial settings. The input data for bfLEAP™ can include raw data (preclinical and/or clinical readouts), categorical data, sociodemographic data of patients, and various other inputs. Thus, the bfLEAP™ platform is capable of capturing the particular genetic and physical characteristics of patients in an unbiased manner, and contextualizing it against other disparate data sources from patients (e.g. molecular data, physiological data, etc.) for less biased and more meaningful conclusions. It is also uniquely scalable - the bfLEAP™ platform is able to perform analysis on large, high-volume data sets (i.e. ‘big data’) and also able to analyze highly disparate “short and wide” data as well. In terms of visualization, bfLEAP™ is able to integrate with most commonly used visualization tools for graph analytics.
We believe that the combination of a) scalable analytics (i.e., large data or short/wide data), b) state-of-the-art proprietary algorithms, c) unsupervised machine learning, and d) streamlined data ingestion/visualization makes bfLEAP™ one of the most flexible and powerful new platforms available on the market.
The Company will continue to evolve and improve bfLEAP™, and some of the proceeds from this offering may be used toward that effort either in-house or with development partners like The Johns Hopkins University Applied Physics Lab.
Summary Risk Factors
Our business is subject to numerous risks as described in the section entitled “Risk Factors” and elsewhere in this prospectus. You should carefully consider these risks before making an investment. Some of these risks include:
| ● | We have a limited operating history upon which you can evaluate our performance, and accordingly, our prospects must be considered in light of the risks that any new company encounters. | |
| ● | In order for the Company to compete and grow, it must attract, recruit, retain and develop the necessary personnel who have the needed experience. | |
| ● | The development and commercialization of our technology, products, and services is highly competitive. | |
| ● | The Company’s success depends on the experience and skill of the board of directors, its executive officers and key employees. | |
| ● | We rely on various intellectual property rights, including patents and licenses in order to operate our business. | |
| ● | From time to time, third parties may claim that one or more of our products or services infringe their intellectual property rights. | |
| ● | New product development involves a lengthy, expensive and complex process. | |
| ● | We may not be able to conduct clinical trials necessary to commercialize and sell our proposed products and formulations. | |
| ● | Our long-term viability and growth will depend upon successful clinical trials. | |
| ● | We face significant competition from other biotechnology and pharmaceutical companies. | |
| ● | Our research and development efforts may not succeed in developing commercially successful products and technologies, which may limit our ability to achieve profitability. | |
| ● | Even if we are able to obtain regulatory approvals for new pharmaceutical products, generic or branded, the success of those products is dependent upon acceptance of such products, particularly by the pharmaceutical industry. |
| 3 |
| ● | We extensively outsource our clinical trial activities and usually perform only a small portion of the start-up activities in-house. | |
| ● | We may not be able to acquire the rights to any failed drugs or we may not be able to rescue failed drugs through analysis due to our technology or the lack of clinical data. | |
| ● | We have no current specific plan for a significant portion of the offering proceeds and it is possible that the proceeds will be invested in a way that does not yield a favorable, or any, return for you. |
Implications of Being an Emerging Growth Company
As a company with less than $1.235 billion in revenue during our last completed fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may take advantage of specified reduced reporting requirements that are otherwise applicable generally to public companies. These reduced reporting requirements include:
| ● | an exemption from compliance with the auditor attestation requirement on the effectiveness of our internal control over financial reporting; | |
| ● | an exemption from compliance with any requirement that the Public Company Accounting Oversight Board may adopt regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements; | |
| ● | an exemption from the requirements to obtain a non-binding advisory vote on executive compensation or a stockholder approval of any golden parachute arrangements; | |
| ● | extended transition periods for complying with new or revised accounting standards; | |
| ● | being permitted to present only two years of audited financial statements and only two years of related “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, in addition to any required unaudited interim financial statements in this prospectus; and | |
| ● | reduced disclosures regarding executive compensation in our periodic reports, proxy statements and registration statements, including in this prospectus. |
We will remain an emerging growth company until the earliest to occur of: (i) the end of the first fiscal year in which our annual gross revenue is $1.235 billion or more; (ii) the end of the first fiscal year in which we are deemed to be a “large accelerated filer,” as defined in the Securities Exchange Act of 1934, as amended, (the “Exchange Act”); (iii) the date on which we have, during the previous three-year period, issued more than $1.00 billion in non-convertible debt securities; and (iv) the end of the fiscal year during which the fifth anniversary of this offering occurs. We may choose to take advantage of some, but not all, of the available benefits under the JOBS Act. We currently intend to take advantage of the exemptions discussed above. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock.
We are also a “smaller reporting company,” as defined under SEC Regulation S-K. As such, we also are exempt from the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and also are subject to less extensive disclosure requirements regarding executive compensation in our periodic reports and proxy statements. We will continue to be deemed a smaller reporting company until our public float exceeds $75 million on the last day of our second fiscal quarter in the preceding fiscal year.
| 4 |
Corporate Information
Bullfrog AI Holdings, Inc. was incorporated in the State of Nevada on February 6, 2020. Bullfrog AI Holdings, Inc. is the parent company of Bullfrog AI, Inc. and Bullfrog AI Management, LLC. which were incorporated in Delaware and Maryland, in 2017 and 2021, respectively. All of our operations are currently conducted through BullFrog AI Holdings, Inc. The Company’s principal business address is 325 Ellington Blvd, Unit 317, Gaithersburg, MD 20878. Our website address is www.bullfrogai.com. The references to our website in this prospectus are inactive textual references only. The information on our website is neither incorporated by reference into this prospectus nor intended to be used in connection with this offering. All of our operations are currently conducted through BullFrog AI, Inc.
Going Concern
The Company intends to overcome the circumstances that impact its ability to remain a going concern through a combination of expanding its revenues and additional equity and debt financing. The Company anticipates raising additional funds through public or private financing, strategic relationships or other arrangements in the near future to support its business operations; however, the Company may not have commitments from third parties for a sufficient amount of additional capital. The Company cannot be certain that any such financing will be available on acceptable terms, or at all, and its failure to raise capital when needed could limit its ability to continue its operations. The Company’s ability to obtain additional funding will determine its ability to continue as a going concern. Failure to secure additional financing in a timely manner and on favorable terms would have a material adverse effect on the Company’s financial performance, results of operations and stock price and may require it to curtail or cease operations, sell off its assets, seek protection from its creditors through bankruptcy proceedings, or otherwise. Furthermore, additional equity financing may be dilutive to the holders of the Company’s common stock, and debt financing, if available, may involve restrictive covenants, and strategic relationships, if necessary, to raise additional funds, and may require that the Company relinquish valuable rights. Please see note 1, in our financial statements, for further information. The Company believes that, upon the closing of this offering, which is expected to be February 5, 2024, it will have sufficient capital to sustain its operations for at least the next 15 months, however, there can be no assurance that sufficient funds required during the subsequent year or thereafter will be generated from operations or that funds will be available from external sources such as debt or equity financings or other potential sources.
THE OFFERING
The following summary of the offering contains basic information about the offering and the common stock and is not intended to be complete. It does not contain all the information that is important to you. For a more complete understanding of the common stock, please refer to the section of this prospectus entitled “Description of Capital Stock.”
| Securities offered by us | We are offering (i) 1,028,710 shares (the “Company Shares”) of our common stock, par value $0.00001 per share, and accompanying warrants to purchase up to 1,028,710 shares of common stock (“Company Warrants”) at a public offering price of $3.782 per Company Share and accompanying Company Warrant, and (ii)478,429 pre-funded warrants (“Pre-Funded Warrants”) to purchase up to 478,429 shares of common stock and 478,429 accompanying Company Warrants at a public offering price of $3.871 per Pre-Funded Warrant and accompanying Company Warrant. | |
| Company Warrants | Each warrant has an exercise price of $4.16 per share of common stock are immediately exercisable and will expire five years from the date of issuance. Please see “Description of Capital Stock - Company Warrants” for a description of these warrants. | |
| Pre-Funded Warrants | We are also offering 478,429 Pre-Funded Warrants to purchase up to 478,429 shares of our common stock, exercisable at an exercise price of $0.001 per share, to those purchasers whose purchase of common stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering. The purchase price of each Pre-funded Warrant and accompanying Company Warrant is equal to $3.781, or the price per share of common stock being sold to the public in this offering minus $0.001. The Pre-funded Warrants will be immediately exercisable and may be exercised at any time until exercised in full Please see “Description of Capital Stock - Pre-Funded Warrants” for a description of these warrants. | |
| Public Offering Price | $3.782 per Company Share and accompanying Company Warrant, or $3.781 per Pre-Funded Warrant and accompanying Company Warrant | |
| Nasdaq Listing | Our common stock and tradeable warrants currently trade on the Nasdaq Capital Market under the symbol “BFRG” and “BFRGW,” respectively. | |
| Number of shares of common stock outstanding before the Offering: | 6,094,644 shares | |
| Number of shares of common stock outstanding after the Offering: | 7,601,783 shares (assuming the exercise of all 478,429 pre-funded Warrants and no exercise of warrants issued in this Offering and the Representative does not exercise its over-allotment option) | |
| Over-allotment option | We have granted the underwriters an option, exercisable within 45-days after the closing of this Offering to purchase an additional 226,071 Company Shares and/or 226,071 Pre-Funded Warrants and/or 226,071 Company Warrants from us, representing 15% of the securities sold in this Offering. |
| 5 |
| Underwriter | WallachBeth Capital LLC | |
| Underwriter’s Warrants | Upon the closing of this Offering, which is expected to be February 5, 2024, we have agreed to issue to the Representative of the underwriters warrants (“Underwriter’s Warrants”) to purchase 90,428 shares of our common stock, equal to six percent (6%) of the aggregate number of shares sold in the Offering. The Underwriter’s Warrants will be exercisable at any time, and from time to time, in whole or in part, during the period commencing 180 days following the effective date of this Offering and expiring on the fifth anniversary thereof at an exercise price per share of $4.16 (110% of the combined public offering price of each Company Share and Company Warrant). See “Underwriting - Underwriter’s Warrants”. The registration statement of which this prospectus is apart also covers the Underwriter’s Warrants and the shares of our common stock issuable upon exercise of the Underwriter’s Warrants. | |
| Use of proceeds: | We expect to receive approximately $4,933,000 in net proceeds from the sale of our securities offered by us in this Offering (approximately $5,710,195 if the underwriters exercise their over-allotment option in full) upon the closing of this Offering, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. We intend to use the net proceeds received from this Offering for general and working capital purposes, including but not limited to investing in research and development, including in our technology, the repayment of debt and for working capital and general corporate purposes.
See “Use of Proceeds” on page 22 for a description of the intended use of proceeds from this Offering. | |
| Risk Factors: | Investing in our securities is highly speculative and involves a high degree of risk. You should carefully consider the information set forth in this prospectus and, in particular, the specific factors set forth in the “Risk Factors” section beginning on page 7 of this prospectus before deciding whether or not to invest in our securities. | |
| Lock-ups | We and our directors, officers and holders of ten percent (10%) or more of our outstanding securities have agreed with the underwriters, subject to certain exceptions, not to offer for sale, issue, sell, contract to sell, pledge or otherwise dispose of any of our common stock for a period of ninety (90) days after the completion of this Offering. See “Underwriting” on page 60. |
Unless otherwise indicated in this prospectus, the information in this prospectus assumes:
| ● | A public offering price of $3.782 per Company Share and accompanying Company Warrant; and | |
| ● | no exercise of the Company Warrants being offered in this Offering, no exercise of the underwriters’ over-allotment option, and no exercise of the Underwriter’s Warrants. |
| 6 |
RISK FACTORS
An investment in our securities is highly speculative and involves a high degree of risk. In determining whether to purchase the Company’s securities, an investor should carefully consider all of the material risks described below, together with the other information contained in this prospectus. We cannot assure you that any of the events discussed below will not occur. These events could have a material and adverse impact on our business, financial condition, results of operations and prospects. If that were to happen, the trading price of our common stock could decline, and you could lose all or part of your investment.
Risks Related to Liquidity, the Company’s Business and Industry
We have a limited operating history upon which you can evaluate our performance, and accordingly, our prospects must be considered in light of the risks that any new company encounters.
We were incorporated under the laws of Nevada on February 26, 2020. Accordingly, we have no significant history upon which an evaluation of our prospects and future performance can be made. Our proposed operations are subject to all of the business risks associated with a new enterprise. The likelihood of our creation of a viable business must be considered in light of the problems, expenses, difficulties, complications, and delays frequently encountered in connection with the inception of a business, operation in a competitive industry, and the continued development of our technology and the results of our clinical data. We anticipate that our operating expenses will increase for the near future. There can be no assurances that we will ever operate profitably. You should consider the Company’s business, operations and prospects in light of the risks, expenses and challenges faced as an early-stage company.
If we are unable to attract and retain key management, scientific personnel and advisors, we may not achieve our business objectives.
Our success depends on the availability and contributions of members of our senior management team. The loss of services of any of these individuals could delay, reduce or prevent our drug development and other business objectives. Furthermore, recruiting and retaining qualified scientific personnel to perform drug development work will be critical to our success. We face intense competition for qualified individuals from numerous pharmaceutical and biotechnology companies, universities, governmental entities and other public and private research institutions. We may be unable to attract and retain these individuals, and our failure to do so could materially adversely affect our business and financial condition.
| 7 |
The development of our technology, products, and services is highly competitive.
We face competition with respect to any products that we may seek to develop or commercialize in the future. Our competitors include major companies worldwide. Many of our competitors have significantly greater financial, technical and human resources than we have and superior expertise in research and development and marketing approved products/services and thus may be better equipped than us to develop and commercialize products/services. These competitors also compete with us in recruiting and retaining qualified personnel and acquiring technologies. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Accordingly, our competitors may commercialize products more rapidly or effectively than we are able to, which would adversely affect our competitive position, the likelihood that our products/services will achieve initial market acceptance and our ability to generate meaningful additional revenues from our products.
From time to time, third parties may claim that one or more of our products or services infringe their intellectual property rights.
Any dispute or litigation regarding patents or other intellectual property could be costly and time consuming due to the uncertainty of intellectual property litigation and could divert our management and key personnel from our business operations. A claim of intellectual property infringement could force us to enter into a costly or restrictive license agreement, which might not be available under acceptable terms or at all, could require us to redesign our products, which would be costly and time-consuming, and/or could subject us to an injunction against development and sale of certain of our products or services. We may have to pay substantial damages, including damages for past infringement if it is ultimately determined that our products infringe on a third party’s proprietary rights. Even if these claims are without merit, defending a lawsuit takes significant time, may be expensive and may divert management’s attention from other business concerns. Any public announcements related to litigation or interference proceedings initiated or threatened against us could cause our business to be harmed. Our intellectual property portfolio may not be useful in asserting a counterclaim, or negotiating a license, in response to a claim of intellectual property infringement. In certain of our businesses we rely on third party intellectual property licenses and we cannot ensure that these licenses will be available to us in the future on favorable terms or at all.
Although dependent on certain key personnel, the Company does not have any key man life insurance policies on any such people.
The Company is dependent on Vininder Singh in order to conduct its operations and execute its business plan and the loss of Vininder Singh or any member of the board of directors or executive officer could harm the Company’s business, financial condition, cash flow and results of operations; however, the Company has not purchased any insurance policies with respect to those individuals in the event of their death or disability. Therefore, if Vininder Singh or any member of the board of directors or an executive officer dies or become disabled, the Company will not receive any compensation to assist with such person’s absence. The loss of such person could negatively affect the Company and its operations.
| 8 |
New product development involves a lengthy, expensive and complex process.
We may be unable to develop or commercialize any product candidates. Moreover, even if we develop such candidates, they may be subject to significant regulatory review, approval and other government regulations. There can be no assurance that our technologies will be capable of developing and commercializing products at all. New product development involves a lengthy, expensive and complex process and we currently have no fully validated diagnostic candidates. In addition, before we can commercialize any new product candidates, we will need to:
| ● | conduct substantial research and development; | |
| ● | conduct validation studies; | |
| ● | expend significant funds; | |
| ● | develop and scale-up our laboratory processes; and | |
| ● | obtain regulatory approval and acceptance of our product candidates. |
This process involves a high degree of risk and takes several years. Our product development efforts may fail for many reasons, including:
| ● | failure of the product at the research or development stage; and | |
| ● | lack of clinical validation data to support the effectiveness of the product. |
Few research and development projects result in commercial products, and perceived viability in early clinical trials often is not replicated in later studies. At any point, we may abandon development of a product candidate or we may be required to expend considerable resources repeating clinical trials, which would adversely impact the timing for generating potential revenues from those product candidates. In addition, as we develop product candidates, we will have to make significant investments in product development, marketing and sales resources.
We may not be able to conduct clinical trials necessary to increase the value of our proposed products and formulations.
In order to conduct clinical trials that are necessary to obtain approval of a product by the FDA, it is necessary to receive clearance from the FDA to conduct such clinical trials. The FDA can halt clinical trials at any time for safety reasons or because we or our clinical investigators do not follow the FDA’s requirements for conducting clinical trials. If we are unable to receive clearance to conduct clinical trials or the trials are halted by the FDA, the likelihood of our ability to sell or license our products would be greatly reduced as it is the FDA approval which will enhance the value of our products.
Our ability to resell and/or license our products will depend upon successful clinical trials.
Only a small number of research and development programs result in the development of a product that obtains FDA approval. Success in preclinical work or early stage clinical trials does not ensure that later stage or larger scale clinical trials will be successful. Conducting clinical trials is a complex, time-consuming and expensive process. Our ability to complete our clinical trials in a timely fashion depends in large part on a number of key factors including protocol design, regulatory and institutional review board approval, the rate of patient enrollment in clinical trials, and compliance with extensive current Good Clinical Practices. If we fail to adequately manage the design, execution and regulatory aspects of our clinical trials, our studies and ultimately our regulatory approvals may be delayed, or we may fail to gain approval for our product candidates. Clinical trials may indicate that our product candidates have harmful side effects or raise other safety concerns that may significantly reduce the likelihood of regulatory approval, result in significant restrictions on use and safety warnings in any approved label, adversely affect placement within the treatment paradigm, or otherwise significantly diminish the commercial potential of the product candidate. Also, positive results in a registrational trial may not be replicated in any subsequent confirmatory trials. Even if later stage clinical trials are successful, regulatory authorities may disagree with our view of the data or require additional studies, and may fail to approve or delay approval of our product candidates or may grant marketing approval that is more restricted than anticipated, including indications for a narrower patient population than expected and the imposition of safety monitoring or educational requirements or risk evaluation and mitigation strategies. In addition, if another Company is the first to file for marketing approval of a competing drug candidate, that Company may ultimately receive marketing exclusivity for its drug candidate, thereby reducing the value of our product.
| 9 |
We face significant competition from other biotechnology and pharmaceutical companies.
While we believe that our technology, development experience and scientific knowledge provide competitive advantages, we face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical, and biotechnology companies, academic institutions and governmental agencies, and public and private research institutions. Many of our existing or potential competitors have substantially greater financial, technical and human resources than we do and significantly greater experience in the development of drug candidates as well as in obtaining regulatory approvals of those drug candidates in the United States and in foreign countries.
Mergers and acquisitions in the pharmaceutical and biotechnology industries could result in even more resources being concentrated among a small number of our competitors. Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in these industries. Our competitors may succeed in developing, acquiring or licensing, on an exclusive basis, drug candidates that are more effective or less costly than any drug candidate that we may develop.
Our ability to compete successfully will depend largely on our ability to:
| ● | identify drugs that have suffered set backs in the clinical development and regulatory process which we believe can be assisted by our platform’s ability to design a better study group; |
| ● | attract qualified scientific, product development and commercial personnel; |
| ● | obtain patent or other proprietary protection for our drugs and technologies; |
| ● | obtain required regulatory approvals; successfully collaborate with pharmaceutical companies in the discovery, development and commercialization of new drugs; and |
| ● | negotiate competitive pricing and reimbursement with third party payors |
The availability of our competitors’ technologies could limit the demand, and the price we are able to charge for our services and for any drug candidate we develop. The inability to compete with existing or subsequently introduced drug development technologies would have a material adverse impact on our business, financial condition and prospects.
Established pharmaceutical companies and research institutions may invest heavily to accelerate discovery and development of novel compounds or to in license novel compounds that could make bfLEAP™ less competitive, which would have a material adverse impact on our business.
We may not be able to acquire the rights to any failed drugs or we may not be able to rescue failed drugs through analysis due to our technology or the lack of clinical data.
Our business model is based on the use of AI/ML technology, which technology may not uncover actionable insights or we may not be able to access sufficient clinical data to uncover such insights that lead to a successful project, clinical trial, or product. The failure of such projects, clinical trials or products would result in a loss of revenue from one of our three sources, which could have a material adverse impact on our business as a whole.
We may not succeed in acquiring the rights to failed drugs, which could limit one of our main sources of revenue.
Our business model is partly based on our ability to acquire drugs that have failed to pass Phase 2 or Phase 3 of the FDA approval process; however, there is no guarantee that we will be able to acquire the rights to such drugs, which would significantly impact our ability to generate revenue and as a result would have a material adverse impact on our business.
We intend to invest in early stage experimental technologies which have a high risk of failure.
To continue supporting our business model, we intend to invest in early stage and experimental technologies, some or all of which may not be useful to us. There is a risk that we will invest in technology that will not ultimately contribute to the success of our projects, which could have a material adverse impact on our business.
We are dependent on our collaborative agreements for the development of products and business development, which exposes us to the risk of reliance on the viability of third parties.
In conducting our research and development activities, we currently rely, and will in the future rely, on collaborative agreements with third parties such as manufacturers, contract research organizations, commercial partners, universities, governmental agencies and not-for-profit organizations for both strategic and financial resources. The loss of, or failure to perform by us or our partners under, any applicable agreements or arrangements, or our failure to secure additional agreements for other products in development, would substantially disrupt or delay our research and development and commercialization activities. Any such loss would likely increase our expenses and materially harm our business, financial condition and results of operation.
| 10 |
We extensively outsource our clinical trial activities and usually perform only a small portion of the start-up activities in-house.
We rely on independent third-party contract research organizations (CROs) to perform most of our clinical studies, including document preparation, site identification, screening and preparation, pre-study visits, training, program management and bioanalytical analysis. Many important aspects of the services performed for us by the CROs are out of our direct control. If there is any dispute or disruption in our relationship with our CROs, our clinical trials may be delayed. Moreover, in our regulatory submissions, we rely on the quality and validity of the clinical work performed by third-party CROs. If any of our CROs’ processes, methodologies or results were determined to be invalid or inadequate, our own clinical data and results and related regulatory approvals could be adversely impacted.
We are a biotechnology company with no significant revenue. We have incurred operating losses since our inception, and we expect to incur losses for the foreseeable future and may never achieve profitability.
We have incurred significant operating losses since our inception. To date, we have not generated any revenue and we may not generate any revenue from sales of our clinical analytics services or drug candidates for the foreseeable future. We expect to continue to incur significant operating losses and we anticipate that our losses may increase substantially as we expand our drug development programs.
To achieve profitability, we must successfully develop and obtain regulatory approval for one or more of drugs and effectively commercialize any drugs we develop. Even if we succeed in developing and commercializing one or more drug candidates, we may not be able to generate sufficient revenue and we may never be able to achieve or sustain profitability.
We will continue to require additional capital for the foreseeable future. If we are unable to raise additional capital when needed, we may be forced to delay, reduce or eliminate our drug acquisition efforts.
We expect to continue to incur significant operating expenses in connection with our ongoing activities, including conducting clinical trials and seeking regulatory approval of drug candidates. Our ongoing future capital requirements will depend on numerous factors, including:
| ● | the rate of progress, results and costs of completion of clinical trials of drug candidates; | |
| ● | the size, scope, rate of progress, results and costs of completion of any potential future clinical | |
| ● | trials and preclinical tests of our drug candidates that we may initiate; | |
| ● | the costs of obtaining regulatory approval of drug candidates; | |
| ● | the scope, prioritization and number of drug development programs we pursue; | |
| ● | the costs for preparing, filing, prosecuting, maintaining and enforcing our intellectual property | |
| ● | rights and defending intellectual property-related claims; | |
| ● | the extent to which we acquire or in-license other products and technologies and the costs to be able to obtain regulatory approval of such products; | |
| ● | our ability to establish strategic collaborations and licensing or other arrangements on terms | |
| ● | favorable to us; and | |
| ● | competing technological and market developments. |
Any additional fundraising efforts may divert our management from their day to day activities, which may adversely affect our ability to identify and acquire new drug candidates and to further the regulatory process of such products. Our ability to raise additional funds will depend, in part, on the success of our product development activities and other factors related to financial, economic and market conditions, many of which are beyond our control. There can be no assurance that we will be able to raise additional capital when needed or on terms that are favorable to us, if at all. If adequate funds are not available on a timely basis, we may be forced to:
| ● | delay, reduce the scope of or eliminate one or more of our drug development programs; |
| 11 |
| ● | limit the amount of new products that we acquire or relinquish, license or otherwise dispose of rights on terms that are less favorable than if we were able to further the regulatory approval process; or | |
| ● | liquidate and dissolve the Company. |
If our operating plans change, we may require additional capital sooner than planned. Such additional financing may not be available when needed or on terms favorable to us. In addition, we may seek additional capital due to favorable market conditions or strategic considerations, even if we believe we have sufficient funds for our current and future operating plan.
We are increasingly dependent on information technology systems to operate our business and a cyber-attack or other breach of our systems, or those of third parties on whom we may rely, could subject us to liability or interrupt the operation of our business.
We are increasingly dependent on information technology systems to operate our business. A breakdown, invasion, corruption, destruction or interruption of critical information technology systems by employees, others with authorized access to our systems or unauthorized persons could negatively impact operations. In the ordinary course of business, we collect, store and transmit confidential information and it is critical that we do so in a secure manner to maintain the confidentiality and integrity of such information. Additionally, we outsource certain elements of our information technology systems to third parties. As a result of this outsourcing, our third party vendors may or could have access to our confidential information making such systems vulnerable. Data breaches of our information technology systems, or those of our third party vendors, may pose a risk that sensitive data may be exposed to unauthorized persons or to the public. For example, the loss of clinical trial data from completed or ongoing clinical trials or preclinical studies could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. While we believe that we have taken appropriate security measures to protect our data and information technology systems, and have been informed by our third party vendors that they have as well, there can be no assurance that our efforts will prevent breakdowns or breaches in our systems, or those of our third party vendors, that could materially adversely affect our business and financial condition.
We must complete extensive clinical trials to demonstrate the safety and efficacy of our drug candidates. If we are unable to demonstrate the safety and efficacy of our drug candidates, we will not be successful.
The success of our business depends primarily on our ability to further the regulatory approval process to increase the value of our drug candidates. Drug candidates must satisfy rigorous standards of safety and efficacy before they can be approved for sale which greatly enhances their value. To satisfy these standards, we must engage in expensive and lengthy testing of drug candidates.
We may not be able to obtain authority from the FDA or other equivalent foreign regulatory agencies to move on to further efficacy segments of the Phase 2 or Phase 3 clinical trials or commence and complete any clinical trials for any of our drug candidates. Positive results in preclinical studies of a drug candidate may not be predictive of similar results in human clinical trials, and promising results from early clinical trials of a drug candidate may not be replicated in later clinical trials. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials even after achieving promising results in early-stage development. Accordingly, the results from the preclinical tests or clinical trials for our drug candidates may not be predictive of the results we may obtain in later stage trials. The failure of clinical trials to demonstrate safety and efficacy of one or more of our drug candidates will have a material adverse effect on our business and financial condition.
Delays in the commencement of clinical trials of our drug candidates could result in increased costs to us and delay our ability to successfully license or sell such products.
Our drug candidates will require continued extensive clinical trials to increase the value and desirability of the products. Because of the nature of clinical trials, we do not know whether future planned clinical trials will begin on time, if at all. Delays in the commencement of clinical trials could significantly increase our drug development costs and delay our ability to successfully sell or license our drug candidates. In addition, many of the factors that may cause, or lead to, a delay in the commencement of clinical trials may also ultimately lead to denial of regulatory approval of a drug candidate. The commencement of clinical trials can be delayed for a variety of reasons, including delays in:
| ● | demonstrating sufficient safety and efficacy in past clinical trials to obtain regulatory approval | |
| ● | to commence a further clinical trial; |
| 12 |
| ● | convincing the FDA that we have selected valid endpoints for use in proposed clinical trials; and | |
| ● | obtaining institutional review board approval to conduct a clinical trial at a prospective site. |
In addition, the commencement of clinical trials may be delayed due to insufficient patient enrollment, which is a function of many factors, including the size of the patient population, the nature of the protocol, the proximity of patients to clinical sites, the availability of effective treatments for the relevant disease and the eligibility criteria for the clinical trial.
If we are unable to obtain U.S. and/or foreign regulatory approval, we will be unable to resell or license our drug candidates.
Our drug candidates will be subject to extensive governmental regulations relating to, among other things, research, testing, development, manufacturing, safety, efficacy, record keeping, labelling, marketing and distribution of drugs. Rigorous preclinical testing and clinical trials and an extensive regulatory approval process are required in the U.S. and in many foreign jurisdictions prior to the commercial sale of drug candidates. Satisfaction of these and other regulatory requirements is costly, time consuming, uncertain and subject to unanticipated delays. It is possible that no drug candidate that we present to the FDA will obtain marketing approval which will significantly diminish the value and desirability of our product candidates. In connection with the clinical trials for our drug candidates, we face risks that:
| ● | the drug candidate may not prove to be efficacious; | |
| ● | the drug candidate may not prove to be safe; | |
| ● | the drug candidate may not be readily co-administered or combined with other drugs or drug | |
| ● | candidates; | |
| ● | the results may not confirm the positive results from earlier preclinical studies or clinical trials; | |
| ● | the results may not meet the level of statistical significance required by the FDA or other | |
| ● | regulatory agencies; and | |
| ● | the FDA or other regulatory agencies may require us to carry out additional studies. |
We have limited experience in conducting and managing later stage clinical trials necessary to obtain regulatory approvals, including approval by the FDA. However, this risk would be mitigated in the event the Company is successful entering into a co-development agreement with a pharma partner for late stage clinical development. The time required to complete clinical trials and for the FDA and other countries’ regulatory review processes is uncertain and typically takes many years. Our analysis of data obtained from preclinical and clinical trials is subject to confirmation and interpretation by regulatory authorities, which could delay, limit or prevent regulatory approval. We may also encounter unanticipated delays or increased costs due to government regulation from future legislation or administrative action or changes in FDA policy during the period of product development, clinical trials, and FDA regulatory review.
We will rely on third parties for manufacturing of our clinical drug supplies; our dependence on these manufacturers may impair the development of our drug candidates.
We have no ability to internally manufacture the drug candidates that we need to conduct our clinical trials for the products that we acquire. For the foreseeable future, we expect to continue to rely on third-party manufacturers and other third parties to produce, package and store sufficient quantities of our drug candidates and any future drug candidates for use in our clinical trials. We may face various risks and uncertainties in connection with our reliance on third-party manufacturers, including:
| ● | reliance on third-party manufactures for regulatory compliance and quality assurance; | |
| ● | the possibility of breach of the manufacturing agreement by the third-party manufacturer because | |
| ● | of factors beyond our control; | |
| ● | the possibility of termination or nonrenewal of our manufacturing agreement by the third-party | |
| ● | manufacturer at a time that is costly or inconvenient for us; | |
| ● | the potential that third-party manufacturers will develop know-how owned by such third-party | |
| ● | manufacturer in connection with the production of our drug candidates that is necessary for the | |
| ● | manufacture of our drug candidates; and | |
| ● | reliance on third-party manufacturers to assist us in preventing inadvertent disclosure or theft of | |
| ● | our proprietary knowledge. |
| 13 |
Our drug candidates may be complicated and expensive to manufacture. If our third-party manufacturers fail to deliver our drug candidates for clinical use on a timely basis, with sufficient quality, and at commercially reasonable prices, we may be required to delay or suspend clinical trials or otherwise discontinue development of our drug candidates. While we may be able to identify replacement third-party manufacturers or develop our own manufacturing capabilities for these drug candidates, this process would likely cause a delay in the availability of our drug candidates and an increase in costs. In addition, third-party manufacturers may have a limited number of facilities in which our drug candidates can be manufactured, and any interruption of the operation of those facilities due to events such as equipment malfunction or failure or damage to the facility by natural disasters could result in the cancellation of shipments, loss of product in the manufacturing process or a shortfall in available drug candidates.
We may rely on technology solution partners for the development and deployment of our AI technology
Our partners may experience technical, financial, operational, or security issues that reduce or eliminate their ability to support the Company. This could prevent the Company from generating revenue and eliminate our ability to operate.
In addition to the risks listed above, businesses are often subject to risks not foreseen or fully appreciated by the management. It is not possible to foresee all risks that may affect us. Moreover, the Company cannot predict whether the Company will successfully effectuate the Company’s current business plan. Each prospective Purchaser is encouraged to carefully analyze the risks and merits of an investment in the Securities and should take into consideration when making such analysis, among other, the Risk Factors discussed above.
Risks Related to Intellectual Property Rights
We rely on various intellectual property rights, including patents and licenses in order to operate our business.
Our intellectual property rights, may not be sufficiently broad or otherwise may not provide us a significant competitive advantage. In addition, the steps that we have taken to maintain and protect our intellectual property may not prevent it from being challenged, invalidated, circumvented or designed-around, particularly in countries where intellectual property rights are not highly developed or protected. In some circumstances, enforcement may not be available to us because an infringer has a dominant intellectual property position or for other business reasons, or countries may require compulsory licensing of our intellectual property. Our failure to obtain or maintain intellectual property rights that convey competitive advantage, adequately protect our intellectual property or detect or prevent circumvention or unauthorized use of such property, could adversely impact our competitive position and results of operations. We also rely on nondisclosure and noncompetition agreements with employees, consultants and other parties to protect, in part, trade secrets and other proprietary rights. There can be no assurance that these agreements will adequately protect our trade secrets and other proprietary rights and will not be breached, that we will have adequate remedies for any breach, that others will not independently develop substantially equivalent proprietary information or that third parties will not otherwise gain access to our trade secrets or other proprietary rights.
As we expand our business, protecting our intellectual property will become increasingly important. The protective steps we have taken may be inadequate to deter our competitors from using our proprietary information. In order to protect or enforce our patent rights, we may be required to initiate litigation against third parties, such as infringement lawsuits. Also, these third parties may assert claims against us with or without provocation. These lawsuits could be expensive, take significant time and could divert management’s attention from other business concerns. The law relating to the scope and validity of claims in the technology field in which we operate is still evolving and, consequently, intellectual property positions in our industry are generally uncertain. We cannot assure you that we will prevail in any of these potential suits or that the damages or other remedies awarded, if any, would be commercially valuable.
| 14 |
The Company could be negatively impacted if found to have infringed on intellectual property rights.
Technology companies, including many of the Company’s competitors, frequently enter into litigation based on allegations of patent infringement or other violations of intellectual property rights. In addition, patent holding companies seek to monetize patents they have purchased or otherwise obtained. As the Company grows, the intellectual property rights claims against it will likely increase. The Company intends to vigorously defend infringement actions in court and before the U.S. International Trade Commission. The plaintiffs in these actions frequently seek injunctions and substantial damages. Regardless of the scope or validity of such patents or other intellectual property rights, or the merits of any claims by potential or actual litigants, the Company may have to engage in protracted litigation. If the Company is found to infringe one or more patents or other intellectual property rights, regardless of whether it can develop non-infringing technology, it may be required to pay substantial damages or royalties to a third-party, or it may be subject to a temporary or permanent injunction prohibiting the Company from marketing or selling certain products. In certain cases, the Company may consider the desirability of entering into licensing agreements, although no assurance can be given that such licenses can be obtained on acceptable terms or that litigation will not occur. These licenses may also significantly increase the Company’s operating expenses. Regardless of the merit of particular claims, litigation may be expensive, time-consuming, disruptive to the Company’s operations and distracting to management. In recognition of these considerations, the Company may enter into arrangements to settle litigation. If one or more legal matters were resolved against the Company’s consolidated financial statements for that reporting period could be materially adversely affected. Further, such an outcome could result in significant compensatory, punitive or trebled monetary damages, disgorgement of revenue or profits, remedial corporate measures or injunctive relief against the Company that could adversely affect its financial condition and results of operations.
We rely heavily on our technology and intellectual property, but we may be unable to adequately or cost-effectively protect or enforce our intellectual property rights, thereby weakening our competitive position and increasing operating costs.
To protect our rights in our services and technology, we rely on a combination of copyright and trademark laws, patents, trade secrets, confidentiality agreements and protective contractual provisions. We also rely on laws pertaining to trademarks and domain names to protect the value of our corporate brands and reputation. Despite our efforts to protect our proprietary rights, unauthorized parties may copy aspects of our services or technology, obtain and use information, marks, or technology that we regard as proprietary, or otherwise violate or infringe our intellectual property rights. In addition, it is possible that others could independently develop substantially equivalent intellectual property. If we do not effectively protect our intellectual property, or if others independently develop substantially equivalent intellectual property, our competitive position could be weakened.
Effectively policing the unauthorized use of our services and technology is time-consuming and costly, and the steps taken by us may not prevent misappropriation of our technology or other proprietary assets. The efforts we have taken to protect our proprietary rights may not be sufficient or effective, and unauthorized parties may copy aspects of our services, use similar marks or domain names, or obtain and use information, marks, or technology that we regard as proprietary. We may have to litigate to enforce our intellectual property rights, to protect our trade secrets, or to determine the validity and scope of others’ proprietary rights, which are sometimes not clear or may change. Litigation can be time consuming and expensive, and the outcome can be difficult to predict.
We rely on agreements with third parties to provide certain services, goods, technology, and intellectual property rights necessary to enable us to implement some of our applications.
Our ability to implement and provide our applications and services to our clients depends, in part, on services, goods, technology, and intellectual property rights owned or controlled by third parties. These third parties may become unable to or refuse to continue to provide these services, goods, technology, or intellectual property rights on commercially reasonable terms consistent with our business practices, or otherwise discontinue a service important for us to continue to operate our applications. If we fail to replace these services, goods, technologies, or intellectual property rights in a timely manner or on commercially reasonable terms, our operating results and financial condition could be harmed. In addition, we exercise limited control over our third-party vendors, which increases our vulnerability to problems with technology and services those vendors provide. If the services, technology, or intellectual property of third parties were to fail to perform as expected, it could subject us to potential liability, adversely affect our renewal rates, and have an adverse effect on our financial condition and results of operations.
If any third-party owners of intellectual property we may license in the future do not properly maintain or enforce the patents underlying such licenses, our competitive position and business prospects will be harmed.
We may enter into licenses for third-party intellectual property in the future. Our success will depend in part on the ability of our licensors to obtain, maintain and enforce patent protection for their intellectual property, in particular, those patents to which we have secured exclusive rights.
| 15 |
If applicable, our licensors may not successfully prosecute the patent applications to which we are licensed. Even if patents issue in respect of any such patent applications, our licensors may fail to maintain these patents, may determine not to pursue litigation against other companies that are infringing these patents, or may pursue such litigation less aggressively than we would. In addition, our licensors may terminate their agreements with us in the event we breach the applicable license agreement and fail to cure the breach within a specified period of time. Without protection for the intellectual property we license, other companies might be able to offer substantially identical products for sale, which could materially adversely affect our competitive business position, business prospects and financial condition.
Because our research and development of drug candidates often incorporates compounds and other information that is the intellectual property of third parties, we depend on continued access to such intellectual property to conduct and complete our preclinical and clinical research and commercialize the drug candidates that result from this research. We expect that future licenses would impose, numerous obligations on us. For example, under our existing and future license agreements, we may be required to pay (i) annual maintenance fees until a drug candidate is sold for the first time, (ii) running royalties on net sales of drug candidates, (iii) minimum annual royalties after a drug candidate is sold for the first time, and (iv) one-time payments upon the achievement of specified milestones. We may also be required to reimburse patent costs incurred by the licensor, or we may be obligated to pay additional royalties, at specified rates, based on net sales of our drug candidates that incorporate the licensed intellectual property rights. We may also be obligated under some of these agreements to pay a percentage of any future sublicensing revenues that we may receive. Future license agreements may also include payment obligations such as milestone payments or minimum expenditures for research and development. We expect that any future licenses would contain reporting, insurance and indemnification requirements. We are actively reviewing and preparing additional patent applications to expand our patent portfolio, but there can be no assurances that patents related to our existing patent applications or any applications we may file in the future will be issued or that any issued patents will provide meaningful protection for our drug candidates, which could materially adversely affect our competitive business position, business prospects and financial condition.
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information and may not adequately protect our intellectual property.
We rely on trade secrets to protect our technology, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. In order to protect our proprietary technology and processes, we also rely in part on confidentiality and intellectual property assignment agreements with our corporate partners, employees, consultants, outside scientific collaborators and sponsored researchers and other advisors. These agreements may not effectively prevent disclosure of confidential information nor result in the effective assignment to us of intellectual property, and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information or other breaches of the agreements. In addition, others may independently discover our trade secrets and proprietary information, and in such case we could not assert any trade secret rights against such party. Enforcing a claim that a party illegally obtained and is using our trade secrets is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, courts outside the U.S. may be less willing to protect trade secrets. Costly and time-consuming litigation could be necessary to seek to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could materially adversely affect our business and financial condition.
Risks Related to Ownership of Our Securities and this Offering
Our management will have broad discretion over the use of any net proceeds from this offering and you may not agree with how we use the proceeds, and the proceeds may not be invested successfully.
Our management will have broad discretion as to the use of any net proceeds from this offering and could use them for purposes other than those contemplated at the time of this offering. Accordingly, you will be relying on the judgment of our management with regard to the use of any proceeds from this offering and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. We have no current specific plan for a significant portion of the offering proceeds and it is possible that the proceeds will be invested in a way that does not yield a favorable, or any, return for you.
Investors in this offering may experience future dilution as a result of this and future equity offerings.
In order to raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock. Investors purchasing our shares or other securities in the future could have rights superior to existing common stockholders, and the price per share at which we sell additional shares of our common stock or other securities convertible into or exchangeable for our common stock in future transactions may be higher or lower than the price per share in this offering.
| 16 |
The price of our common stock may fluctuate substantially.
You should consider an investment in our common stock to be risky, and you should invest in our common stock only if you can withstand a significant loss and wide fluctuations in the market value of your investment. Some factors that may cause the market price of our common stock to fluctuate, in addition to the other risks mentioned in this “Risk Factors” section and elsewhere in this prospectus, are:
| ● | sales of our common stock by our stockholders, executives, and directors; |
| ● | volatility and limitations in trading volumes of our shares of common stock; |
| ● | our ability to obtain financing to conduct and complete research and development activities; |
| ● | our ability to attract new customers; |
| ● | changes in the development status of the drugs we acquire; |
| ● | failures to meet external expectations or management guidance; |
| ● | changes in our capital structure or dividend policy or future issuances of securities; |
| ● | our cash position; |
| ● | announcements and events surrounding financing efforts, including debt and equity securities; |
| ● | reputational issues; |
| ● | announcements of acquisitions, partnerships, collaborations, joint ventures, new products, capital commitments, or other events by us or our competitors; |
| ● | changes in general economic, political and market conditions in or any of the regions in which we conduct our business; |
| ● | changes in industry conditions or perceptions; |
| ● | changes in valuations of similar companies or groups of companies; |
| ● | analyst research reports, recommendation and changes in recommendations, price targets, and withdrawals of coverage; |
| ● | departures and additions of key personnel; |
| ● | disputes and litigations related to intellectual property rights, proprietary rights, and contractual obligations; |
| ● | changes in applicable laws, rules, regulations, or accounting practices and other dynamics; and |
| ● | other events or factors, many of which may be out of our control. |
| 17 |
In addition, if the market for stocks in our industry or industries related to our industry, or the stock market in general, experiences a loss of investor confidence, the trading price of our common stock could decline for reasons unrelated to our business, financial condition and results of operations. If any of the foregoing occurs, it could cause our stock price to fall and may expose us to lawsuits that, even if unsuccessful, could be costly to defend and a distraction to management.
We do not intend to apply for any listing of the Pre-Funded Warrants, Company Warrants or Underwriter’s Warrants on any exchange or nationally recognized trading system, and we do not expect a market to develop for the Pre-Funded Warrants, Company Warrants or Underwriter’s Warrants.
We do not intend to apply for any listing of either of the Pre-Funded Warrants, Company Warrants or Underwriter’s Warrants (collectively, the “Warrants”) on the Nasdaq Capital Market or any other securities exchange or nationally recognized trading system, and we do not expect a market to develop for the Warrants. Without an active market, the liquidity of the Warrants will be limited. Further, the existence of the Warrants may act to reduce both the trading volume and the trading price of our common stock.
The Warrants are speculative in nature and holders of Warrants have no rights as stockholders until such holders exercise their Warrants and acquire our shares of common stock.
Until holders of our Warrants acquire shares of common stock upon exercise thereof, such holders will have no rights with respect to the shares of common stock underlying the Warrants. Upon exercise of the Warrants, the holders will be entitled to exercise the rights of a stockholder only as to matters for which the record date occurs after the date they were entered in the register of members of the Company as a stockholder. Following this Offering, the market value of the Warrants is uncertain. There can be no assurance that the market price of our common stock will ever equal or exceed the exercise price of the Company Warrants and the Underwriter’s Warrants or the price of the Pre-Funded Warrants, and, consequently, whether it will ever be profitable for investors to exercise their Warrants.
Future sales of shares by existing stockholders could cause our stock price to decline.
If our existing stockholders sell, or indicate an intent to sell, substantial amounts of our common stock in the public market after the ninety (90) day contractual lock-up and other legal restrictions on resale discussed in this prospectus lapse, the trading price of our common stock could decline significantly and could decline below the public offering price. Based on shares outstanding as of the date of this prospectus, upon the completion of this offering, we will have 7,123,354 outstanding shares of common stock (assuming none of the warrants or pre-funded warrants issued in this offering are exercised), assuming the underwriter does not exercise its over-allotment option and none of the Warrants issued in this Offering are exercised. Of these shares, assuming no shares are purchased in this offering by our existing stockholders, 4,443,437 shares of common stock, will be immediately freely tradable, without restriction, in the public market.
In addition, upon issuance, the 900,000 shares reserved for future issuance under our 2022 Equity Incentive Plan may become eligible for sale in the public market in the future, subject to certain legal and contractual limitations. If our existing stockholders sell substantial amounts of our common stock in the public market, or if the public perceives that such sales could occur, this could have an adverse impact on the market price of our common stock, even if there is no relationship between such sales and the performance of our business.
After the completion of this offering, we may be at an increased risk of securities class action litigation.
Historically, securities class action litigation has often been brought against a company following a decline in the market price of its securities. If we were to be sued, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
We have never paid dividends on our capital stock and we do not anticipate paying any dividends in the foreseeable future. Consequently, any gains from an investment in our common stock will likely depend on whether the price of our common stock increases.
We have not paid dividends on any of our classes of capital stock to date and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. In addition, the terms of any future indebtedness we may incur could preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain from an investment in our common stock for the foreseeable future. Consequently, in the foreseeable future, you will likely only experience a gain from your investment in our common stock if the price of our common stock increases.
| 18 |
If equity research analysts do not publish research or reports about our business or if they issue unfavorable commentary or downgrade our common stock, the price of our common stock could decline.
The trading market for our common stock may be affected by the research and reports that equity research analysts publish about us and our business. We do not control these analysts. The price of our common stock could decline if one or more equity analysts downgrade our common stock or if analysts issue other unfavorable commentary or cease publishing reports about us or our business.
Provisions of our charter documents or Nevada law could delay or prevent an acquisition of our company, even if the acquisition would be beneficial to our stockholders, and could make it more difficult to change management.
Provisions of our articles of incorporation and bylaws may discourage, delay or prevent a merger, acquisition or other change in control that stockholders might otherwise consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. In addition, these provisions may frustrate or prevent any attempt by our stockholders to replace or remove our current management by making it more difficult to replace or remove our board of directors. These provisions include:
| ● | limitations on our stockholders’ ability to call special meetings of stockholders; | |
| ● | an advance notice requirement for stockholder proposals and nominations for members of our | |
| ● | Board; | |
| ● | the authority of our Board to determine the number of director seats on our Board; | |
| ● | the authority of our Board to fill vacancies occurring on the Board; | |
| ● | the authority of our Board to issue preferred stock with such terms as our Board may determine. |
Our articles of incorporation grants our Board of Directors the power to designate and issue additional shares of common and/or preferred stock.
Our authorized capital consists of 100,000,000 shares of common stock and 10,000,000 shares of preferred stock. Our preferred stock may be designated into series pursuant to authority granted by our articles of incorporation, and on approval from our Board of Directors. The Board of Directors, without any action by our stockholders, may designate and issue shares in such classes or series as the Board of Directors deems appropriate and establish the rights, preferences and privileges of such shares, including dividends, liquidation and voting rights. The rights of holders of other classes or series of stock that may be issued could be superior to the rights of holders of our common stock. The designation and issuance of shares of capital stock having preferential rights could adversely affect other rights appurtenant to shares of our common stock.
We will indemnify and hold harmless our officers and directors to the maximum extent permitted by Nevada law.
Our bylaws provide that we will indemnify and hold harmless our officers and directors against claims arising from our activities, to the fullest extent not prohibited by Nevada law. If we were called upon to perform under our indemnification agreement, then the portion of our assets expended for such purpose would reduce the amount otherwise available for our business.
| 19 |
We are an “emerging growth company” under the JOBS Act of 2012 and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and we may take advantage of certain exemptions from various reporting requirements that are not applicable to other public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
In addition, Section 107 of the JOBS Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933 (the “Securities Act”) for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We are choosing to take advantage of the extended transition period for complying with new or revised accounting standards.
We will remain an “emerging growth company” until the last day of the fiscal year following the fifth anniversary of the date of the first sale of our common stock pursuant to an effective registration statement under the Securities Act, although we will lose that status sooner if our revenues exceed $1.07 billion, if we issue more than $1 billion in non-convertible debt in a three year period, or if the market value of our common stock that is held by non-affiliates exceeds $700 million as of the last day of our most recently completed second fiscal quarter.
Investors may be unable to compare our business with other companies in our industry if they believe that our financial accounting is not as transparent as other companies in our industry. If we are unable to raise additional capital as and when we need it, our financial condition and results of operations may be materially and adversely affected.
Because Vininder Singh, our Chief Executive Officer and director, controls a significant number of shares of our voting capital stock, he has effective control over actions requiring stockholder approval.
Mr. Vininder Singh, our Chief Executive Officer and a director, beneficially owns approximately 43% of the Company’s common stock. As a result, Mr. Singh possesses significant influence on the outcome of matters submitted to our stockholders for approval, including the election of directors and any merger, consolidation or sale of all or substantially all of our assets. Accordingly, any investors who purchase shares will be minority shareholders and as such will have little to no say in the direction of us and the election of directors. Additionally, this concentration of ownership might harm the market price of our common stock by:
| ● | delaying, deferring or preventing a change in corporate control; |
| ● | impeding a merger, consolidation, takeover or other business combination involving us; or |
| ● | discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us. |
| 20 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that involve substantial risks and uncertainties. The forward-looking statements are contained principally in the sections entitled “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business,” but are also contained in this prospectus. In some cases, you can identify forward-looking statements by the words “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “aim,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing,” “target,” “seek” or the negative of these terms, or other comparable terminology intended to identify statements about the future. Forward-looking statements contained in this prospectus include, but are not limited to, statements about:
| ● | our future financial performance, including our revenue, costs of revenue, operating expenses and profitability; |
| ● | the sufficiency of our cash and cash equivalents to meet our liquidity needs; |
| ● | our predictions about the property development, digital transformation technology and biohealth businesses and their respective market trends; |
| ● | our ability to attract and retain customers in all our business segments to purchase our products and services; |
| ● | the availability of financing for smaller publicly traded companies like us; |
| ● | our ability to successfully expand in our three principal business markets and into new markets and industry verticals; and |
| ● | our ability to effectively manage our growth and future expenses. |
We caution you that the foregoing list may not contain all of the forward-looking statements made in this prospectus.
These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained in this prospectus, we caution you that these statements are based on a combination of facts and factors currently known by us and our expectations of the future, about which we cannot be certain.
You should refer to the “Risk Factors” section of this prospectus for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result, of these factors, we cannot assure you that the forward-looking statements in this prospectus will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by federal securities law.
You should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
| 21 |
USE OF PROCEEDS
We estimate that the net proceeds from our issuance and sale of Company Shares and accompanying Common Warrants and Pre-funded Warrants and accompanying Company Warrants will be approximately $4,933,000 from this Offering. If the underwriter exercises its over-allotment option in full, we estimate that our net proceeds will be approximately $5,710,195, after deducting the underwriter discount and estimated offering expenses payable by us.
As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to us from the Offering. Accordingly, we will retain broad discretion over the use of these proceeds, if any. Generally, we intend to use the net proceeds received from this Offering for general and working capital purposes, including but not limited to investing in research and development, including in our technology.
DIVIDEND POLICY
Holders of common stock are entitled to receive ratably such dividends, if any, as may be declared by the Board of Directors out of funds legally available. We have not paid any dividends since our inception, and we presently anticipate that all earnings, if any, will be retained for development of our business. Any future disposition of dividends will be at the discretion of our Board of Directors and will depend upon, among other things, our future earnings, operating and financial condition, capital requirements, and other factors.
CAPITALIZATION
The following table sets forth our cash, cash equivalents, capitalization and indebtedness as of September 30, 2023:
| ● | on an actual basis; and | |
| ● | on a pro forma as adjusted basis to give effect to the sale of the 1,028,710 Company Shares and accompanying Company Warrant at a combined public offering price of $3.782 and 478,429 Pre-Funded Warrants and accompanying Company Warrant at a combined public offering price of $3.781, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
| 22 |
The pro forma information below is illustrative only. You should read the following table in conjunction with the “Use of Proceeds” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of this prospectus and our consolidated financial statements and related notes appearing elsewhere in this prospectus.
| September 30, 2023 (Unaudited) | ||||||||
| Actual | Pro Forma(1)(2) | |||||||
| Cash and cash equivalents | $ | 3,856,037 | $ | 8,789,037 | ||||
| Debt | $ | 446,050 | $ | 446,050 | ||||
| Stockholders deficit: | ||||||||
| Series A Convertible Preferred Stock, $0.00001 par value, 5,500,000 shares authorized; 73,449 shares issued and outstanding (actual); 73,449 issued and outstanding (pro forma) | 1 | 1 | ||||||
| Common Stock, $0.00001 par value, 100,000,000 shares authorized; 6,094,644 issued and outstanding (actual); 7,601,783 issued and outstanding (pro forma) | 61 | 76 | ||||||
| Additional paid-in capital | 12,226,742 | 17,159,727 | ||||||
| Accumulated deficits | (8,457,978 | ) | (8,457,978 | ) | ||||
| Total stockholders’ deficit | 3,768,826 | 9,147,876 | ||||||
| Total Capitalization | $ | 3,768,826 | $ | 9,147,876 | ||||
| (1) | Reflects the sale of Company Shares in this Offering at a public offering price of $3.782 and sale of Company Pre-Funded Warrants at a public offering price of $3.781, and after deducting the estimated underwriting discounts, and estimated offering expenses payable by us. The pro forma information is illustrative only, and we will adjust this information based on the actual public offering price and other terms of this offering which were determined at pricing. |
| (2) | If the underwriters’ option to purchase up to an additional 226,071 Company Shares and/or Company Warrants, in any combination thereof, is exercised we would receive approximately $777,195 in additional net proceeds, based on the assumed combined public offering price of $3.782 per Company Share and Company Warrant, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us; and (ii) cash and cash equivalents, total shareholders’ equity and total capitalization would each also increase by approximately $777,195. |
The total number of shares of our common stock reflected in our actual and pro forma information set forth in the table above excludes:
| ● | Warrants to purchase 2,893,954 shares of common stock issued as part of the IPO unit at an average exercise price of $7.96 per share, which expire on February 13, 2028; | |
| ● | Warrants to purchase 108,849 shares of common stock at an exercise price of $5.29 per share, with terms expiring April 1, 2026 through February 13, 2028; | |
| ● | Warrants to purchase 403,890 shares of common stock at an exercise price of $2.64 per share, with terms expiring April 1, 2026 through May 3, 2032; | |
| ● | Options to purchase 438,500 shares of common stock at a weighted average exercise price of $4.41 per share; | |
| ● | Warrants to purchase 274,284 shares of common stock at an exercise price of $0.0007 per share, with terms expiring February 7, 2030; and | |
| ● | Warrants to purchase 115,185 shares of common stock at an exercise price of $2.50 per share, with terms expiring August 19, 2031. |
DILUTION
If you’ve purchased Company Shares and Company Warrants in this Offering, your ownership interest will be diluted to the extent of the difference between the combined public offering price per Company Share and accompanying Company Warrant and the pro forma as adjusted net tangible book value per share of our common stock immediately upon the closing of this Offering. Some of the information that we provide in this discussion is on a pro forma basis to give further effect to our issuance and sale of 1,028,710 Company Shares and accompanying Company Warrants at a combined public offering price of $3.782 per Company Share and Company Warrant and 478,429 Pre-Funded Warrant and accompanying Company Warrant at a combined public offering price of $3.781 per Pre-Funded Warrant and accompanying Company Warrant, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us (assuming the issuance of the 478,429 shares underlying the pre-funded warrants).
The net tangible book value of our common stock as of September 30, 2023, was $3,768,826 or $0.62 per share. Net tangible book value per share represents our total tangible assets (which excludes deferred offering costs, which were $0 at September 30, 2023, less our total liabilities, divided by the number of shares of outstanding common stock).
| 23 |
After giving further effect to the receipt of the net proceeds from our sale of 1,028,710 Company Shares and accompanying Company Warrants in this Offering, at a combined public offering price of $3.782 per Company Share and Company Warrant and 478,429 Pre-Funded Warrant and accompanying Company Warrant at a combined public offering price of $3.781 per Pre-Funded Warrant and accompanying Company Warrant, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma net tangible book value as of September 30, 2023 would have been approximately $8,701,826 or $1.14 per share. This amount represents an immediate increase in pro forma net tangible book value of $0.52 per share to our existing stockholders and an immediate dilution of $2.64 per share to new investors who have purchased Company Shares in this Offering.
We determine dilution per share to investors participating in this Offering by subtracting pro forma as adjusted net tangible book value per share after the closing of this Offering from the public offering price per share paid by investors that have participated in this Offering. The following table illustrates this dilution on a per share basis to new investors:
| Public offering price per Company Share and Company Warrant | $ | 3.782 | ||
| Net tangible book value per share as of September 30, 2023 | $ | 0.62 | ||
| Pro forma net tangible book value (deficit) per share as of September 30, 2023 | $ | 1.14 | ||
| Increase in pro forma as adjusted net tangible book value per share attributable to new investors purchasing shares | $ | 0.52 | ||
| Pro forma as adjusted net tangible book value per share after this offering | $ | 1.14 | ||
| Dilution in net tangible book value per share to new investors in this Offering | $ | 2.64 |
Each $1.00 increase (decrease) in the combined public offering price of $3.782 per Company Share and Company Warrant would increase (decrease) the pro forma net tangible book value by 1,507,139 per share and increase (decrease) the dilution per share to new investors by ($1.00) per share, assuming the number of Company Shares and accompanying Company Warrants offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated underwriting discounts and commissions.
Similarly, each increase (decrease) of 100,000 Company Shares and accompany Company Warrants we’ve offered would increase (decrease) our pro forma net tangible book value by approximately $344,162, or $0.63 per share and decrease (increase) the dilution per share to new investors participating in this offering by $2.54 per share, and after deducting the estimated underwriting discounts and commissions.
If the underwriters exercise their option to purchase additional Company Shares and/or Company Warrants in this Offering in full at the combined public offering price of $3.782 per Company Share and Company Warrant and 478,429 Pre-Funded Warrant and accompanying Company Warrant at a combined public offering price of $3.781 per Pre-Funded Warrant and accompanying Company Warrant and assuming the number of Company Shares and accompanying Company Warrants offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting estimated underwriting discounts and commissions and estimated offering expenses, the pro forma net tangible book value would be approximately $1.21 per share, and the dilution in pro forma net tangible book value per share to investors in this offering would be approximately $2.57 per share.
The table below summarizes as of September 30, 2023, adjusted pro forma basis described above, the number of shares of our common stock, the total consideration and the average price per share (i) paid to us by existing stockholders and (ii) to be paid by new investors who purchased our common stock in this offering at a combined public offering price of $3.782 per Company Share and Company Warrant, before deducting underwriting discounts and commissions and estimated offering expenses.
| Shares Purchased | Total Consideration | Average Price Per | ||||||||||||||||||
| Number | Percent | Amount | Percent | Share | ||||||||||||||||
| Existing stockholders | 6,094,644 | 80.2 | % | $ | 12,224,742 | 68.2 | % | $ | 2.01 | |||||||||||
| New investors | 1,507,139 | 19.8 | % | $ | 5,700,000 | 31.8 | % | $ | 3.782 | |||||||||||
| Total | 7,601,783 | 100.0 | % | $ | 17,924,742 | 100.0 | % | $ | 2.36 | |||||||||||
In addition, if the underwriters exercise their option to purchase additional Company Shares and/or Company Warrants in full, the number of shares held by existing stockholders will be reduced to 3.1% of the total number of shares of common stock to be outstanding upon the closing of this Offering, and the number of shares of common stock held by new investors who have participated in this Offering will be further increased by 226,071, or 3.1% of the total number of shares of common stock to be outstanding upon the closing of this Offering.
| 24 |
Each $1.00 increase (decrease) in the combined public offering price of $3.782 per Company Share and Company Warrant would increase (decrease) total consideration paid by new investors by approximately $777,195, assuming that the number of Company Shares and accompanying Company Warrants offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated underwriting discounts and commissions.
The total number of shares of our common stock reflected in our actual and pro forma information set forth in the table above excludes:
| ● | Warrants to purchase 2,893,954 shares of common stock issued as part of the IPO unit at an average exercise price of $7.96 per share, which expire on February 13, 2028; | |
| ● | Warrants to purchase 108,849 shares of common stock at an exercise price of $5.29 per share, with terms expiring April 1, 2026 through February 13, 2028; | |
| ● | Warrants to purchase 403,890 shares of common stock at an exercise price of $2.64 per share, with terms expiring April 1, 2026 through May 3, 2032; | |
| ● | Options to purchase 638,500 shares of common stock at a weighted average exercise price of $4.21 per share; | |
| ● | Warrants to purchase 274,284 shares of common stock at an exercise price of $0.0007 per share, with terms expiring February 7, 2030; and | |
| ● | Warrants to purchase 115,185 shares of common stock at an exercise price of $2.50 per share, with terms expiring August 19, 2031. |
The discussion and table above assume no exercise of the Warrants. To the extent that the Warrants are exercised, any outstanding warrants or options are exercised, any outstanding convertible notes are converted any outstanding restricted stock units vest, or new options or restricted stock units are issued our equity incentive plan, you may experience further dilution. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
DESCRIPTION OF CAPITAL STOCK
The following description of the material terms of our capital stock and the provisions of our amended and restated articles of incorporation and bylaws are summaries and are qualified by reference to copies of the amended and restated articles of incorporation and bylaws, which are filed as exhibits to our registration statement of which this prospectus forms a part.
General
Our authorized capital stock consists of 100,000,000 shares of common stock, par value $0.00001 per share, and 10,000,000 shares of preferred stock, par value $0.00001 per share, including 5,500,000 shares of Series A Preferred Stock.
Common Stock
Common stock outstanding
As of January 25, 2024, there were 6,094,644 shares of common stock outstanding.
Voting rights
Each share of common stock entitles the holder to one vote, either in person or by proxy, at meetings of stockholders. The holders are not permitted to vote their shares cumulatively.
Dividend rights
Holders of common stock are entitled to receive ratably such dividends, if any, as may be declared by the Board of Directors out of funds legally available.
Rights upon liquidation
Upon our liquidation, dissolution or winding up, the holders of our common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities.
Other rights
Holders of our common stock do not have any pre-emptive rights or other subscription rights, conversion rights, redemption or sinking fund provisions.
| 25 |
Preferred Stock
Preferred stock outstanding
As of January 25, 2024, there are 73,449 shares of Series A Preferred Stock issued and outstanding.
Conversion rights
Each holder of Series A Preferred Stock may, from time to time, convert any or all of such holder’s shares of Series A Preferred Stock into fully paid and nonassessable shares of common stock in an amount equal to ten shares of common stock for each one share of Series A Preferred Stock surrendered.
A holder of shares of Series A Preferred Stock is not entitled to convert shares of Series A Preferred Stock if upon such conversion the number of shares of common stock to be received, together with the number of shares of common stock beneficially owned by the holder and its affiliates on the conversion date, would result in beneficial ownership by the bolder and its affiliates of more than 4.99% of the outstanding shares of common stock of the Company on such conversion date
Voting rights
Each holder of Series A Preferred Stock has no voting rights.
Rights upon liquidation
Upon our liquidation, dissolution or winding up, the holders of our Series A Preferred Stock shall not be entitled to any liquidation preference and are to receive any liquidation as if they were converted to common stock.
Warrants
Company Warrants
The registration statement of which this prospectus is a part also registers for sale Company Warrants to purchase up to 1,507,139 shares of common stock (or 1,733,210 shares of common stock if the underwriter exercises its over-allotment option in full).
Each Company Warrant is exercisable for one share of our common stock, with an assumed initial exercise price equal to $4.16 (assuming an exercise price equal to the public offering price per share) per share at any time for up to five (5) years after the date of the closing of this Offering. The Company Warrants issued in this Offering are governed by the terms of the Company Warrants. The holder of a Company Warrant will not be deemed a holder of our underlying common stock until the Company Warrant is exercised.
Subject to certain limitations as described below the Company Warrants are immediately exercisable upon issuance on the closing date and expire on the fifth year anniversary of the closing date. Subject to limited exceptions, a holder of Company Warrants will not have the right to exercise any portion of its Company Warrants if the holder (together with such holder’s affiliates, and any persons acting as a group together with such holder or any of such holder’s affiliates) would beneficially own a number of shares of common stock in excess of 4.99% (or, at the election of the purchaser prior to the date of issuance, 9.99%) of the shares of common stock then outstanding after giving effect to such exercise.
The exercise price and the number of shares issuable upon exercise of the Company Warrants is subject to appropriate adjustment in the event of recapitalization events, stock dividends, stock splits, stock combinations, reclassifications, reorganizations or similar events affecting our common stock. The Company Warrant holders must pay the exercise price in cash upon exercise of the Company Warrants, unless such Company Warrant holders are utilizing the cashless exercise provision of the Company Warrants.
Upon the holder’s exercise of a Company Warrant, we will issue the shares of common stock issuable upon exercise of the Company Warrant within two trading days following our receipt of a notice of exercise, provided that payment of the exercise price has been made (unless exercised to the extent permitted via the “cashless” exercise provision). Prior to the exercise of any Company Warrants to purchase common stock, holders of the Company Warrants will not have any of the rights of holders of the common stock purchasable upon exercise, including the right to vote, except as set forth therein.
Warrant holders may exercise Company Warrants only if the issuance of the shares of common stock upon exercise of the Company Warrants is covered by an effective registration statement, or an exemption from registration is available under the Securities Act and the securities laws of the state in which the holder resides. We intend to use commercially reasonable efforts to have the registration statement, of which this prospectus forms a part, effective when the Company Warrants are exercised. The Company Warrant holders must pay the exercise price in cash upon exercise of the Company Warrants unless there is not an effective registration statement or, if required, there is not an effective state law registration or exemption covering the issuance of the shares underlying the Company Warrants (in which case, the Company Warrants may only be exercised via a “cashless” exercise provision).
In the event we consummate a merger or consolidation with or into another person or other reorganization event in which our common stock are converted or exchanged for securities, cash or other property, or we sell, lease, license, assign, transfer, convey or otherwise dispose of all or substantially all of our assets or we or another person acquire 50% or more of our outstanding shares of common stock, then following such event, the holders of the Company Warrants will be entitled to receive upon exercise of such Company Warrants the same kind and amount of securities, cash or property which the holders would have received had they exercised their Company Warrants immediately prior to such fundamental transaction. Any successor to us or surviving entity shall assume the obligations under the Company Warrants.
We do not intend to apply for listing of the Company Warrants on any securities exchange or other trading system.
| 26 |
Pre-funded Warrants
The term “pre-funded” refers to the fact that the purchase price of our common stock in this Offering includes almost the entire exercise price of $3.781 that will be paid under the Pre-Funded Warrants, except for a nominal remaining exercise price of $0.001. The purpose of the Pre-Funded Warrants is to enable investors that may have restrictions on their ability to beneficially own more than 4.99% (or, upon election of the holder, 9.99%) of our outstanding common stock following the closing of this Offering the opportunity to make an investment in the Company without triggering their ownership restrictions, by receiving Pre-Funded Warrants in lieu of our common stock which would result in such ownership of more than 4.99% (or 9.99%), and receive the ability to exercise their option to purchase the shares underlying the Pre-Funded Warrants at such nominal price at a later date.
Each Pre-Funded Warrant is exercisable for one share of our common stock, with an exercise price equal to $0.001 per share, at any time that the Pre-Funded Warrant is outstanding. There is no expiration date for the Pre-Funded Warrants. The holder of a Pre-Funded Warrant will not be deemed a holder of our underlying common stock until the Pre-Funded Warrant is exercised.
Subject to limited exceptions, a holder of Pre-Funded Warrants will not have the right to exercise any portion of its Pre-Funded Warrants if the holder (together with such holder’s affiliates, and any persons acting as a group together with such holder or any of such holder’s affiliates) would beneficially own a number of shares of common stock in excess of 4.99% (or, at the election of the purchaser prior to the date of issuance, 9.99%) of the shares of our common stock then outstanding after giving effect to such exercise.
The exercise price and the number of shares issuable upon exercise of the Pre-Funded Warrants is subject to appropriate adjustment in the event of recapitalization events, stock dividends, stock splits, stock combinations, reclassifications, reorganizations or similar events affecting our common stock. The Pre-Funded Warrant holders must pay the exercise price in cash upon exercise of the Pre-Funded Warrants, unless such Pre-Funded Warrant holders are utilizing the cashless exercise provision of the Pre-Funded Warrants.
Upon the holder’s exercise of a Pre-Funded Warrant, we will issue the shares of common stock issuable upon exercise of the Pre-Funded Warrant within two trading days following our receipt of a notice of exercise, provided that payment of the exercise price has been made (unless exercised to the extent permitted via the “cashless” exercise provision). Prior to the exercise of any Pre-Funded Warrants to purchase common stock, holders of the Pre-Funded Warrants will not have any of the rights of holders of the common stock purchasable upon exercise, including the right to vote, except as set forth therein.
Warrant holders may exercise Pre-Funded Warrants only if the issuance of the shares of common stock upon exercise of the Pre-Funded Warrants is covered by an effective registration statement, or an exemption from registration is available under the Securities Act and the securities laws of the state in which the holder resides. We intend to use commercially reasonable efforts to have the registration statement, of which this prospectus forms a part, effective when the Pre-Funded Warrants are exercised. The Pre-Funded Warrant holders must pay the exercise price in cash upon exercise of the Pre-Funded Warrants unless there is not an effective registration statement or, if required, there is not an effective state law registration or exemption covering the issuance of the shares underlying the Pre-Funded Warrants (in which case, the Pre-Funded Warrants may only be exercised via a “cashless” exercise provision).
In the event we consummate a merger or consolidation with or into another person or other reorganization event in which our common stock are converted or exchanged for securities, cash or other property, or we sell, lease, license, assign, transfer, convey or otherwise dispose of all or substantially all of our assets or we or another person acquire 50% or more of our outstanding shares of common stock, then following such event, the holders of the Pre-Funded Warrants will be entitled to receive upon exercise of such Pre-Funded Warrants the same kind and amount of securities, cash or property which the holders would have received had they exercised their Pre-Funded Warrants immediately prior to such fundamental transaction. Any successor to us or surviving entity shall assume the obligations under the Pre-Funded Warrants.
We do not intend to apply for listing of the Pre-Funded Warrants on any securities exchange or other trading system.
Underwriter’s Warrants
The registration statement of which this prospectus is a part also registers for sale warrants to purchase up to 90,428 shares of common stock to the Representative of the underwriters as a portion of the underwriting compensation payable to the underwriters in connection with this Offering. The Underwriter’s Warrants will be exercisable during a period commencing at six months from the effective date of the Offering and ending five years from the effective date of the offering at an exercise price of $4.16 per share, equal to 110% of the public offering price of the common stock. Please see “Underwriting - Underwriter’s Warrants” for a description of these warrants.
Warrant Agent
The Company Warrants will be issued in registered form under a warrant agent agreement (the “Warrant Agent Agreement”) between us and our warrant agent, VStock Transfer LLC (the “Warrant Agent”). The material provisions of the the Company Warrants are set forth herein, and a copy of the Warrant Agent Agreement is filed with the SEC as an exhibit to the registration statement of which this prospectus forms a part or are incorporated by reference herein.
Stock Options
As of September 30, 2023, we issued an aggregate of 438,500 stock options. Our directors and Chief Financial Officer were granted stock options to purchase a total of 195,000 additional shares of the Company’s common stock. The options have a 10-year term with an exercise price between $2.80 and $6.82. On April 16, 2023, the Company issued 40,000 stock options with an exercise price of $6.82 per share and a 10-year term to each director, Mr. Elsey, Mr. Enright and Mr. Hanson. On March 17, 2023, the Company issued 75,000 stock options with an exercise price of $2.80 per share and a 10-year term to our Chief Financial Officer, Mr. Saglio. Our employees were granted stock options to purchase a total of 196,500 shares of common stock, which have a 10-year term with an exercise price between $2.80 and $5.45. We also granted to service providers stock options to purchase a total of 67,000 shares of common stock, which have a 10-year term with an exercise price between $2.59 and $5.45. The relative fair value of the 2023 stock options using the Black-Scholes valuation model totals $738,600. As of September 30, 2023, the Company had 461,500 equity-based awards authorized but unissued available under the 2022 Equity Incentive Plan.
Anti-Takeover Provisions of Nevada Law, or Articles of Incorporation and our Bylaws
Our articles of incorporation and bylaws contain certain provisions that may have the effect of delaying, deferring or preventing a party from acquiring control of us and encouraging persons considering unsolicited tender offers or other unilateral takeover proposals to negotiate with our Board of Directors rather than pursue non-negotiated takeover attempts. According to our articles of incorporation and bylaws, neither the holders of our common stock nor the holders of our preferred stock have cumulative voting rights in the election of our directors. The combination of the present ownership by a few stockholders of a significant portion of our issued and outstanding common stock and lack of cumulative voting makes it more difficult for other stockholders to replace our Board of Directors or for a third party to obtain control of our Company by replacing our Board of Directors.
The following provisions of the Nevada Revised Statutes (“NRS”) could, if applicable, have the effect of discouraging takeovers of our Company.
Transactions with Interested Stockholders. The NRS prohibits a publicly-traded Nevada company from engaging in any business combination with an interested stockholder for a period of three years following the date that the stockholder became an interested stockholder unless, prior to that date, the Board of Directors of the corporation approved either the business combination itself or the transaction that resulted in the stockholder becoming an interested stockholder.
| 27 |
An “interested stockholder” is defined as any entity or person beneficially owning, directly or indirectly, 10% or more of the outstanding voting stock of the corporation and any entity or person affiliated with, controlling, or controlled by any of these entities or persons. The definition of “business combination” is sufficiently broad to cover virtually any type of transaction that would allow a potential acquirer to use the corporation’s assets to finance the acquisition or otherwise benefit its own interests rather than the interests of the corporation and its stockholders.
In addition, business combinations that are not approved and therefore take place after the three year waiting period may also be prohibited unless approved by the board of directors and stockholders or the price to be paid by the interested stockholder is equal to the highest of (i) the highest price per share paid by the interested stockholder within the 3 years immediately preceding the date of the announcement of the business combination or in the transaction in which he or she became an interested stockholder, whichever is higher; (ii) the market value per common share on the date of announcement of the business combination or the date the interested stockholder acquired the shares, whichever is higher; or (iii) if higher for the holders of preferred stock, the highest liquidation value of the preferred stock.
Acquisition of a Controlling Interest. The NRS contains provisions governing the acquisition of a “controlling interest” and provides generally that any person that acquires 20% or more of the outstanding voting shares of an “issuing corporation,” defined as Nevada corporation that has 200 or more stockholders at least 100 of whom are Nevada residents (as set forth in the corporation’s stock ledger); and does business in Nevada directly or through an affiliated corporation, may be denied voting rights with respect to the acquired shares, unless a majority of the disinterested stockholder of the corporation elects to restore such voting rights in whole or in part.
The statute focuses on the acquisition of a “controlling interest” defined as the ownership of outstanding shares sufficient, but for the control share law, to enable the acquiring person, directly or indirectly and individually or in association with others, to exercise (i) one-fifth or more, but less than one-third; (ii) one-third or more, but less than a majority; or (iii) a majority or more of the voting power of the corporation in the election of directors.
The question of whether or not to confer voting rights may only be considered once by the stockholders and once a decision is made, it cannot be revisited. In addition, unless a corporation’s articles of incorporation or bylaws provide otherwise (i) acquired voting securities are redeemable in whole or in part by the issuing corporation at the average price paid for the securities within 30 days if the acquiring person has not given a timely information statement to the issuing corporation or if the stockholders vote not to grant voting rights to the acquiring person’s securities; and (ii) if voting rights are granted to the acquiring person, then any stockholder who voted against the grant of voting rights may demand purchase from the issuing corporation, at fair value, of all or any portion of their securities.
The provisions of this section do not apply to acquisitions made pursuant to the laws of descent and distribution, the enforcement of a judgment, or the satisfaction of a security interest, or acquisitions made in connection with certain mergers or reorganizations.
Listing
Our common stock and tradeable warrants are listed on the Nasdaq Capital Market under the symbol “BFRG” and “BFRGW,” respectively. We have not applied, and do not intend to apply, to list the Pre-Funded Warrants or the Company Warrants on the Nasdaq Capital Market.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is VStock Transfer, LLC.
| 28 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the section titled “Selected Consolidated Financial and Other Data” and the consolidated financial statements and related notes thereto included elsewhere in this prospectus. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those discussed below. Factors that could cause or contribute to such differences include, but are not limited to, those identified below and those discussed in the section titled “Risk Factors” included elsewhere in this prospectus.
Overview
Bullfrog AI Holdings, Inc. was incorporated in the State of Nevada on February 6, 2020. Bullfrog AI Holdings, Inc. is the parent company of Bullfrog AI, Inc. and Bullfrog AI Management, LLC, which were incorporated in Delaware and Maryland, in 2017 and 2021, respectively. Operations are currently conducted through Bullfrog AI Holdings, Inc., which began operations on February 6, 2020. We are a company focused specifically on advanced Artificial Intelligence / Machine Learning (AI/ML) analysis of complex data in the advancement of medicine. Our AI/ML platform (trade name: bfLEAP™) was created from technology originally developed at The Johns Hopkins University Applied Physics Laboratory (JHU-APL).
In February 2018, Bullfrog AI Holdings secured the original exclusive, worldwide, royalty-bearing license from JHU-APL for the technology. The license covers three (3)issued patents, one (1) new provisional patent application, non-patent rights to proprietary libraries of algorithms and other trade secrets including modifications and improvements. We entered into a license agreement in July 2022 that provides the Company with new intellectual property and also encompasses most of the intellectual property from the February 2018 license. Our objective is to utilize bfLEAP™ for a precision medicine approach toward drug development with biopharmaceutical collaborators, as well as our own internal clinical development programs. We believe the bfLEAP™ platform is ideally suited for evaluating pre-clinical and clinical trial data generated in translational research and clinical trial settings that lead to faster, less expensive drug approvals.
Our aim is to improve the odds of success in each stage of developing medicine, ranging from early pre-clinical through late-stage clinical development. Our ultimate objective is to utilize bfLEAP™ to enable the success of ongoing clinical trials or rescue late-stage failed drugs (i.e., Phase 2 or Phase 3 clinical trial failures) for development and divestiture; although, we will also consider collaborations for earlier stage drugs. We hope to accomplish this through strategic acquisitions of current clinical stage and failed drugs for in-house development, or through strategic partnerships with biopharmaceutical industry companies.
On July 8, 2022, the Company entered into an exclusive, worldwide, royalty-bearing license from JHU-APL for the additional technology. The new license provides additional intellectual property rights including patents, copyrights, and knowhow to be utilized under the Company’s bfLEAP™ analytical AI/ML platform. In consideration of the new license, the Company issued to JHU-APL 39,879 shares of common stock. In September 2020 and October of 2021, the Company executed amendments to the original license which represents improvements and new advanced analytics capabilities. In consideration of the rights granted to the Company under the original License Agreement, the Company granted JHU 178,571 warrants exercisable to purchase shares of common stock at $2.10 per share. Under the terms of the new License Agreement, JHU will be entitled to eight percent (8%) of net sales for the services provided by the Company to other parties and three percent (3%) for internally developed drug projects in which the JHU license was utilized. The new license also contains tiered sub licensing fees that start at 50% and reduce to 25% based on revenues. On May 31, 2023, the Company and JHU-APL entered into Amendment number 1 of the July 8, 2022 License Agreement whereby the Company gained access to certain improvements including additional patents and knowhow in exchange for a series of payments totaling $275,000. The first of these payments for $75,000 was due in July 2023 followed by annual payments of $75,000, $75,000 and $50,000 in years 2024, 2025 and 2026, respectively. The amendment also reduced the 2023 minimum annual royalty payment to $60,000, all other financial terms remain the same. As a result of this Amendment, the minimum annual payments are set to be $30,000 for 2022, $60,000 for 2023, and $300,000 for 2024 and beyond, all of which are creditable by royalties.
| 29 |
We intend to continue to evolve and improve bfLEAP™, either in-house or with development partners like JHU-APL. We plan to leverage our proprietary AI/ML platform developed over several years at one of the top innovation institutions in the world which has already been successfully applied in multiple sectors.
We have staffed our business using funds from our initial public offering and have entered into partnerships and relationships and recently completed our first commercial service contract with a leading rare disease non-profit organization for AI/ML analysis of late-stage clinical data. We have also acquired the rights to a series of preclinical and early clinical drug assets from universities, as well as a strategic collaboration with a world-renowned research institution to create a HSV1 viral therapeutic platform to engineer immunotherapies for a variety of diseases. We have signed exclusive worldwide License Agreements with JHU for a cancer drug that targets glioblastoma (brain cancer), pancreatic cancer, and others. We have also signed an exclusive worldwide license from George Washington University for another cancer drug that targets hepatocellular carcinoma (liver cancer) and other liver diseases. Additionally, we intend to gain access to later-stage clinical assets through partnerships or the acquisition of rights to failed therapeutic candidates for drug rescue. In certain circumstances, we intend to conduct late-stage clinical trials in an effort to rescue therapeutic assets that previously failed. In these cases, there will be a requirement for drug supply and regulatory services to conduct clinical trials. The success of our clinical development programs will require finding partners to support the clinical development, adequate availability of raw materials and/or drug product for our R&D and clinical trials, and, in some cases, may also require establishment of third-party arrangements to obtain finished drug product that is manufactured appropriately under (GMP) industry-standard guidelines, and packaged for clinical use or sale. Since we are a company focused on using our AI technology to advance medicines, any clinical development programs will also require, in all cases, partners and the establishment of third-party relationships for execution and completion of clinical trials. Over the next 24 months, the Company expects to spend approximately $2.1 million on service offering products, preclinical IND enabling activities and on R&D to enable future clinical trials evaluating our drug assets for new disease indications.
Since completing our IPO on February 14, 2023, aided by the receipt of the IPO proceeds, we have initiated several initiatives: investor relations and marketing to promote and raise awareness of the Company in the financial and business sectors, research and development, collaboration with J. Craig Venter Institute and in the quarter ended September 30, 2023, completed a preclinical study for our Mebendazole prodrug program. The Company is actively engaged in developing and seeking out new intellectual property as it strives to continuously evolve its AI/ML platform. Additionally, the Company has engaged a business development firm specializing in the biopharmaceutical industry to seek and secure a strategic development partner our Mebendazole program.
Internally, the Company has added incremental staff to accelerate execution, and the development of processes and custom scripts for use in performing analytical services for customers, while also launching initiatives targeting large public health data sources and seeking access to proprietary health data sources. We also transitioned our accounting and financial reporting systems and processes to enhance our internal control environment as a public company. Capital from the IPO was also used to retire two notes that were sold to fund the Company through the IPO that did not convert into common stock as well as other debts accrued over time to our staff, employees and consultants as well as obligations related to the acquisition of our licensed drug programs.
| 30 |
Our Strategy
The Company has a unique strategy designed to reduce risk and increase the frequency of cash flow. The first part of the strategy is to generate revenues through strategic relationships with biopharma companies. These relationships will be structured as a combination of fees and intellectual property based on the specific scope of the engagement. The objective of these engagements will be to uncover valuable insights to reduce the risk and/or increase the speed of the drug development process which can be achieved through manual or automated integration into the client’s workflow or analysis of discrete data sets.
In the future, the second part of our strategy involves acquiring the rights to clinical stage drugs, using our bfLEAP™ technology to design a precision medicine trial, conduct the trial with a partner, and sell the asset. This approach may also apply to earlier phases in the drug development process such as discovery and preclinical. In any case, the objective is to create near term value and exit and monetize as quickly as possible, preferably within approximately 30 months.
Critical Accounting Policies and Estimates
Our financial statements are prepared in accordance with U.S. GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue and expenses, as well as related disclosures. We evaluate our estimates and assumptions on an ongoing basis. Our estimates are based on historical experience and various other assumptions that we believe to be reasonable under the circumstances. Our actual results could differ from these estimates. There have been no material changes to our critical accounting policies and estimates as described in our Form 10-K.
Financial Operations Overview
Revenue
While we generated our first revenues in late 2022 from our services provided to a pharmaceutical customer, in Q3 2023 we completed our first commercial service contract and recognized revenue in the amount of $65,000. We have service contracts with two organizations and currently have multiple discussions underway, although there can be no assurance of entering into additional service agreements and business relationships in 2023.
Operating Expenses
We classify our operating expenses into two categories: research and development and general and administrative. Prior to 2022, most of our activities were related to: technology evaluation, acquisition and validation, capital acquisition and business development activities in general, which we believe have readied the Company for contract services while exploring strategic partnering and asset acquisition. These activities and related expenditures have been recorded and reported as General and Administrative in our Financial Statements. In 2022, we licensed two drug development programs from universities and also entered into a new license with JHU-APL for new IP and other enhancements used with our bfLEAP™ platform. In 2022, we expended appropriately $608,000 on license related payments for our bfLEAP™ AI/ML platform and our two drug development programs from universities. We expect that our research and development expenses will increase in 2024 as we initiate activities directed towards the development of service offering products, collaborations (JCVI) and preclinical studies aimed at generating the data to enable the filing of an Investigational New Drug (IND) application.
| 31 |
Research and Development Costs and Expenses
Research and development costs and expenses in 2022 consisted primarily of costs related to the acquisition of licensed technology. In 2023 we have initiated development activities on our licensed drug candidates and our discovery collaboration with JCVI. In addition to fees paid to external service providers, we are also allocating internal costs for personnel working on these efforts in addition to personnel costs related to our internal efforts to develop our product and service offerings using bfLEAP™. We anticipate our research and development costs could become significant as we execute on our business plan and begin conducting preclinical research and development activities directed at securing development partners and filing an IND for our licensed drug development programs described in this filing, as well as under strategic partnerships and for other drug development programs we may acquire. Research and development expenses are recorded in operating expenses in the period in which they are incurred. Estimates will be used in determining the expense liability of certain costs where services have been performed but not yet invoiced. We will monitor levels of performance under each significant contract for external services through communications with the service providers to reflect the actual amount expended.
General and Administrative Expenses
In anticipation of the IPO, a management team with deep industry experience was identified and engaged as employees and consultants to assist the Company in preparing for the IPO and subsequently, to operate and function as a public company. Through 2022, the primary activities included: technology evaluation, acquisition and validation, capital acquisition and business development activities which in general, have readied the Company for contract services while exploring strategic partnering and asset acquisition as noted above. In February 2023, the Company achieved its objective of completing an IPO and listing on NASDAQ. Our 2023 general and administrative expenses are significantly higher than our 2022 general and administrative expenses due to several factors. The primary increases in 2023 relate to new costs associated with being a public company such as D&O insurance, professional services engaged to support SEC compliance as well as higher salary and consulting expenses as we have hired additional staff and consultants. We have also increased our business development, investor relations and marketing efforts. We anticipate that our general and administrative expenses may increase in the future to support our service offerings, clinical and pre-clinical research and development activities associated with strategic partnering and collaborations.
Results of Operations
Comparison of Three Months Ended September 30, 2023 and 2022
Revenue and Costs of Goods Sold
We recognized $65,000 in revenue and $5,200 in costs of goods sold during the three months ended September 30, 2023 versus zero 2022.
Three Months Ended September 30, | Net Change | |||||||||||
| 2023 | 2022 | |||||||||||
| Operating expenses: | ||||||||||||
| Research and development | $ | 380,015 | $ | 39,421 | $ | 340,594 | ||||||
| General and administrative | 983,929 | 601,131 | 382,798 | |||||||||
| Total operating expenses | $ | 1,363,944 | $ | 640,552 | $ | 723,392 | ||||||
Research and Development
Our research and development expenses for the three months ended September 30, 2023 increased by $340,594, compared to the same period ended September 30, 2022, primarily due to the inclusion of the cost of salaries and consulting fees in 2023, as well the cost of acquiring access to additional technology from JHU-APL related to bfLEAP™ pursuant to Amendment 1 of the July 2022 License Agreement. We also completed a preclinical study related to our Mebendazole program in Q3 2023. In 2022, the majority of the research and development expenses were directly related to the acquisition of two drug development product candidates including Mebendazole.
General and Administrative
Our general and administrative expenses for the three months ended September 30, 2023 increased by $382,798, compared to the same period ended September 30, 2022, primarily due to higher salary and consulting costs reflecting an increased level of services as well as the initiation of investor relations and marketing efforts and the transition of our accounting and financial reporting process to support a public company.
| 32 |
Other Income (Expense), Net
Interest expense decreased $118,401 for the three months ended September 30, 2023, compared to the same period ended September 30, 2022 due to the majority of our debt converting or being paid off in the first quarter of 2023. Other income increased by $56,906 due to interest earned on our IPO proceeds which we hold in an overnight sweep account.
Comparison of Nine Months Ended September 30, 2023 and 2022
Revenue and Costs of Goods Sold
We recognized $65,000 in revenue and $5,200 in costs of goods sold during the nine months ended September 30, 2023 versus zero 2022.
| Nine Months Ended September 30, | Net Change | |||||||||||
| 2023 | 2022 | |||||||||||
| Operating expenses: | ||||||||||||
| Research and development | $ | 1,023,619 | $ | 448,375 | $ | 575,244 | ||||||
| General and administrative | 3,067,940 | 1,424,383 | 1,643,557 | |||||||||
| Total operating expenses | $ | 4,091,559 | $ | 1,872,758 | $ | 2,218,801 | ||||||
Research and Development
Our research and development expenses for the nine months ended September 30, 2023 increased by $575,244 compared to the same period ended September 30, 2022, primarily due to the inclusion of the cost of salaries and consulting fees in 2023 as we initiated our collaboration with J Craig Venter Institute and completion of a preclinical study for our Mebendazole prodrug program, as well as the cost of acquiring access to additional technology from JHU-APL related to bfLEAP™ pursuant to Amendment 1 of the July 2022 License Agreement. In 2022, the majority of the research and development expenses were directly related to the acquisition of two drug development product candidates including Mebendazole.
General and Administrative
Our general and administrative expenses for the nine months ended September 30, 2023 increased by $1,643,557, compared to the same period ended September 30, 2022, primarily due to higher salary and consulting costs reflecting an increased level of service as well the initiation of investor relations and marketing efforts and the transition of our accounting and financial reporting process to support a public company. The 2023 period also reflects approximately $120,000 in recruiting fees related to staff additions.
Other Income (Expense), Net
Interest expense decreased $157,788 for the nine months ended September 30, 2023, compared to the same period ended September 30, 2022 due to the majority of our debt converting or being paid off in the first quarter of 2023. The loss on the conversion of notes of $92,959 for the nine months ended September 30, 2023 was due to the conversion of the convertible notes. Other income increased by $142,218 due to interest earned on our IPO proceeds which we hold in an overnight sweep account.
Comparison of the Results of Operations for the years ended December 31, 2022 and 2021
In late 2022, the Company recognized its first service revenues of $10,000 related to an analysis contract with a small pharmaceutical company. The Company previously had not recorded any revenues. Through the end of 2022, the Company has an accumulated deficit of approximately $4,399,000. Net loss from operations in 2022 was approximately $2,455,000 versus $555,000 in 2021. The 2022 increase reflects the full year costs of engaging advisors and consultants and other costs associated with preparing the Company for its initial public offering including the costs related to auditing the Company’s past and current financial statements. Cash used in operations in 2022 was approximately $911,000 versus approximately $382,000 in 2021 and net cash inflows from financing activities in 2022 was approximately $967,000 versus approximately $387,000 in 2021.
| 33 |
Liquidity and Capital Resources
In 2022, the Company received net proceeds from the sale of Convertible Bridge Notes of approximately $1,016,000 and repaid the unsecured promissory notes sold in 2021 in the amount of $49,000. The Company sold one additional promissory note and received net proceeds of $100,000 in January 2023.
For the year ended December 31, 2022, the Company used approximately $911,000 on operating activities versus approximately $382,000 for the same period in 2021. The 2022 cash use included approximately $548,000 in salaries, approximately $634,000 in consulting and professional fees including legal, accounting and auditing fees, as well as consulting fees for operational activities and approximately $609,000 in technology license fees, patent cost reimbursements and minimum annual royalties which has been recorded as a research & development expense.
Through September 30, 2023, the Company has an accumulated deficit of $8,457,978 and funded its operations through the sale of common stock and debt. We anticipate that our expenses will increase in the future to support our service offerings, clinical and pre-clinical research and development activities associated with strategic partnering and collaborations, as well as acquired product candidates and the increased costs of operating as a public company. These increases could include increased costs related to the hiring of additional personnel and fees to outside consultants, lawyers and accountants, among other expenses. Additionally, we anticipate increased costs associated with being a public company including expenses related to services associated with maintaining compliance with exchange listing and Securities and Exchange Commission requirements, insurance, and investor relations costs.
The Company’s current operations include Bullfrog AI, Inc. and Bullfrog Management, LLC, which are wholly owned subsidiaries of Bullfrog AI Holdings, Inc., which is a holding company that depends upon the sale of its securities and cash generated through its subsidiaries to fund consolidated operations.
On February 16, 2023, the Company completed its IPO of 1,297,318 units (each, a “Unit,” collectively, the “Units”) at a price of $6.50 per unit for a total of approximately $8.4 million of gross proceeds to the Company. Each Unit consists of one share of the Company’s common stock, one tradeable warrant (each, a “Tradeable Warrant,” collectively, the “Tradeable Warrants”) to purchase one share of common stock at an exercise price of $7.80 per share, and one non-tradeable warrant (each, a “Non-tradeable Warrant,” collectively, the “Non-tradeable Warrants”; together with the Tradeable Warrants, each, a “Warrant,” collectively, the “Warrants”) to purchase one share of the Company’s common stock at an exercise price of $8.125. In connection with the IPO, the Company also completed a 1-for-7 reverse stock split of our common stock.
In connection with the IPO, a SAFE and convertible loan agreement held by a related party converted into 55,787 shares of post reverse split common stock. Additionally, all outstanding convertible bridge notes and accrued interest through November 30, 2022 were converted into 276,289 shares of common stock and 276,289 warrants to purchase common stock were issued to the Convertible Bridge Note holders at conversion. The convertible bridge note conversions and the warrant exercise pricing was determined using a $25 million dollar company valuation immediately before the IPO.
Between April 5 and April 13, 2023, the holders of warrants exercised 436,533 warrants for common stock at various exercise prices and the Company received proceeds of approximately $1,495,000.
In the absence of significant revenues in 2023, management believes the Company’s capital resources are sufficient to fund planned operations for approximately 9 months from the date of this filing.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, as such term is defined in Item 303(a)(4) of Regulation S-K.
| 34 |
OUR BUSINESS
Our Corporate History and Background
BullFrog AI Holdings, Inc. was incorporated in the State of Nevada on February 18, 2020. Our principal business address is 325 Ellington Blvd, Unit 317, Gaithersburg, MD 20878. All of our operations are currently conducted through BullFrog AI Holdings, Inc. BullFrog AI, Inc., a wholly owned subsidiary acquired through a share exchange, has the sole purpose of housing and protecting all of the organization’s intellectual property. BullFrog AI Management, LLC is a wholly owned subsidiary that handles all HR and payroll activities.
Acquisition of BullFrog AI
In March 2020, BullFrog AI, Inc. received an investment from TEDCO - the Technology Development Corporation of Maryland, a State of Maryland Investment Fund - pursuant to the issuance of a $200,000 convertible note with an 18-month term, 6% annual interest rate, and a 20% discount. In June 2020, BullFrog AI Holdings, Inc. acquired BullFrog AI, Inc. pursuant to an exchange agreement under which each share of Bull Frog AI, Inc. common stock was exchanged for a share of common stock of BullFrog AI Holdings, Inc.. Immediately prior to the share exchange, each outstanding common share of BullFrog AI, Inc. was split into 25 shares of common stock. Share amounts in our financial statements for 2021 and 2020 have been adjusted to reflect this forward share split and shares exchange. Pursuant to the agreement, 24,223,975 shares of the Company’s common stock were issued to the shareholders of BullFrog AI, Inc. in exchange for 100% of the outstanding stock of BullFrog AI, Inc. Upon completion of the exchange, BullFrog AI, Inc. became the Company’s wholly-owned subsidiary and the shareholders of BullFrog AI, Inc. held 100% of the common stock of the Company. As a result, BullFrog AI Holdings, Inc. assumed a total of $330,442 in net liabilities of BullFrog AI, Inc. Both of the entities were controlled before and after the transactions by the same controlling shareholder. This transaction is being accounted for as a common control transaction and all entities are being presented as if the transactions took place at the beginning of the earliest period presented.
BullFrog AI Corporate History
BullFrog AI, Inc. was incorporated in the State of Delaware on August 25, 2017. Vininder Singh is the founder, CEO and chairman of BullFrog AI.
Our Strategy
We plan to achieve our business objectives by enabling the successful development of drugs and biologics using a precision medicine approach via our proprietary artificial intelligence platform bfLEAP. We will execute our plan by doing all or any of the following: partnering with biopharmaceutical companies in a fee for service model to assist and enable them with their drug development programs, acquiring rights to and rescuing drugs that have failed FDA review following pivotal Phase 2 or Phase 3 clinical trials (we refer to this rescue process as “drug rescue”), acquiring rights to drugs that are in early stage clinical trials and have not failed FDA review, and discovering new drugs and biologics.
The process for enhancing and developing late-stage failed drugs is to:
| ● | acquire the rights to the failed drug from a biopharmaceutical industry company or university, | |
| ● | use the proprietary bfLEAP™ AI/ML platform to determine a multi-factorial profile for a patient that would best respond to the drug, | |
| ● | Rapidly conduct a clinical trial likely with a partner to validate the drug’s use for the defined “high-responder” population; and | |
| ● | Divest/sell the rescued drug asset with new information back to the pharma industry, following positive results of the clinical trial. |
We also plan to deploy this strategy for all discovery and early stage clinical candidates. The common objective is to monetize our assets as quickly as possible with no current plan to commercialize any asset. As part of our strategy, we will continue evolving our intellectual property, analytical platform and technologies, build a large portfolio of drug candidates, and implement a model that reduces risk and increases the frequency of cash flow from rescued drugs. This strategy will include strategic partnerships, collaborations, and relationships along the entire business value chain.
| 35 |
We did not produce any revenues through 2021; we generated our first revenues in late 2022 from our services related to the relationship with a pharmaceutic company.
To date, we have not conducted clinical trials on any pharmaceutical drugs and our platform has not been used to identify a drug candidate that has received regulatory approval for commercialization. However, we currently have a strategic relationship with a leading rare disease non-profit organization for artificial intelligence/machine learning (“AI/ML”) analysis of late stage clinical data. We have acquired the rights to a series of preclinical and early clinical drug assets from universities and entered into a strategic collaboration with a world renowned research institution to create a HSV1 viral therapeutic platform to engineer immunotherapies for colorectal cancer. We have signed exclusive worldwide license agreements with Johns Hopkins University for a cancer drug that targets glioblastoma (brain cancer), pancreatic cancer, and other cancers. We have also signed an exclusive worldwide license with George Washington University for another cancer drug that targets hepatoceullar carcinoma (liver cancer), and other liver diseases.
Our platform was originally developed by The Johns Hopkins University Applied Physics Laboratory (“JHU-APL”). JHU-APL uses the same technology for applications related to national defense. Over several years, the software and algorithms have been used to identify relationships, patterns, and anomalies, and make predictions that otherwise may not be found. These discoveries and insights provide an advantage when predicting a target of interest, regardless of industry or sector. We have applied the technology to various clinical data sets and have identified novel relationships that may provide new intellectual property, new drug targets, and other valuable information that may help with patient stratification for a clinical trial thereby improving the odds for success. The platform has not yet aided in the development of a drug that has reached commercialization. However, we have licensed one drug candidate that has completed a Phase 1 trial and a second candidate that is in the preclinical stages. Our aim is to use our technology on current and future available data to help us better determine the optimal path for development.
Contract Services
Our fee for service partnership offering is designed for biopharmaceutical companies, as well as other organizations, of all sizes that have challenges analyzing data throughout the drug development process. We provide the customer with an analysis of large complex data sets using our proprietary artificial intelligence / machine learning platform called bfLEAP™. This platform is designed to predict targets of interest, patterns, relationships, and anomalies. Our service model involves a cash fee plus the potential for rights to new intellectual property generated from the analysis, which can be performed at the discovery, preclinical, or clinical stages of drug development. On September 28, 2022, BullFrog AI entered into a $185,000 service contract with Sapu Biosciences, LLC, a subsidiary of Oncotellic Therapeutics (OTCQB: OTLC). The scope of the contract is focused on uncovering novel insights related to oncology clinical data for one of their candidate programs.
Collaborative Arrangements
We will also seek to enter into collaborative arrangements with pharmaceutical companies who have drugs that have failed late Phase 2 or Phase 3 trials. Our revenue from such collaborations will be based on achieving certain milestones as determined by each specific arrangement.
Acquisition of Rights to Certain Drugs
In certain circumstances, we may also acquire rights to drugs that are in early stage clinical trials, use our technology to produce a successful later stage precision medicine trial, and divest the asset. The same process may apply to the discovery of new drugs.
| 36 |
Our Products
| Product/Platform | Description | Target Market/Indications | ||
| bfLEAP™ - AI/ML platform for analysis of preclinical and/or clinical data | AI/ML analytics platform derived from technology developed at JHU-APL and licensed by the Company. | Biotechnology and pharmaceutical companies and other organizations. | ||
| siRNA | siRNA targeting Beta2-spectrin in the treatment of human diseases developed at George Washington University licensed by the Company | Hepatocellular carcinoma (HCC), treatment of obesity, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis. Has not yet initiated clinical testing. | ||
| Mebendazole | Improved formulation of Mebendazole developed at Johns Hopkins University and licensed by the Company | Glioblastoma. Has begun the process of clinical testing but has not received regulatory approval for commercialization. |
On January 14, 2022, the Company entered into an exclusive, worldwide, royalty-bearing license from George Washington University (GWU) for rights to use siRNA targeting Beta2-spectrin in the treatment of human diseases, including hepatocellular carcinoma (HCC). The license covers methods claimed in three U.S. and worldwide patent applications, and also includes use of this approach for treatment of obesity, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis. This program is currently in the preclinical stage of development. The Company has not yet initiated development activities or IND-enabling studies on this asset; however, the plan is to conduct this work over the next 24 months. All R&D to date on this candidate has been conducted by the licensor of the technology, George Washington University.
Non-alcoholic fatty liver disease (NAFLD) is a condition in which excess lipids, or fat, build up in the liver. This condition, which is more common in people who have obesity and related metabolic diseases including type 2 diabetes, affects as many as 24% of adults in the US and is associated with risk of progression to more serious conditions, including non-alcoholic steatohepatitis (NASH), with associated liver inflammation and fibrosis, and HCC. Evidence in animal models of obesity suggest that a protein called β2-spectrin may play a key role in lipid accumulation, tissue fibrosis, and liver damage, and targeting expression or activity of this protein may be a useful approach in treating NASH and liver cancer (Rao et al., 2021).
In February 2022, the Company entered into an exclusive, worldwide, royalty-bearing license from Johns Hopkins University (JHU) for the use of an improved formulation of Mebendazole for the treatment of any human cancer or neoplastic disease. This formulation shows potent activity in animal models of different types of cancer and has been evaluated in a Phase I clinical trial in patients with high-grade glioma (NCT01729260). The trial, an open-label dose-escalation study, assessed the safety of the improved formulation with adjuvant temozolomide in 24 patients with newly diagnosed gliomas. Investigators observed no dose-limiting toxicity in patients receiving all but the highest tested dose (200mg/kg/day). Four of the 15 patients receiving the maximum tested dose of 200mg/kg/day experienced dose-limiting toxicity, all of which were reversed by decreasing or eliminating the dose given. There were no serious adverse events attributed to mebendazole at any dose during the trial. The Company is currently formulating a strategy to find a partner to conduct additional clinical trials with this asset to enable evaluation of safety in humans.
We are able to leverage our drug rescue business by leveraging a powerful and proven AI/ML platform (trade name: bfLEAP™) initially derived from technology developed at JHU-APL. The bfLEAP™ analytics platform is a potentially disruptive tool for analysis of pre-clinical and/or clinical data sets, such as the robust pre-clinical and clinical trial data sets being generated in translational R&D and clinical trial settings. The input data for bfLEAP™ can include raw data (preclinical and/or clinical readouts), categorical data, sociodemographic data of patients, and various other inputs. Thus, the bfLEAP™ platform is capable of capturing the “human experience” of patients in an unbiased manner, and contextualizing it against other disparate data sources from patients (e.g. molecular data, physiological data, etc.) for less biased and more meaningful conclusions (i.e. more ethical AI/ML). It is also uniquely scalable - the bfLEAP™ platform is able to perform analysis on large, high-volume data sets (i.e. ‘big data’) and also able to analyze highly disparate “short and wide” data as well. In terms of visualization, bfLEAP™ is able to integrate with most commonly used visualization tools for graph analytics.
We believe the combination of a) scalable analytics (i.e., large data or short/wide data), b) state-of-the-art algorithms, c) unsupervised machine learning, and d) streamlined data ingestion/visualization makes bfLEAP™ one of the most flexible and powerful new platforms available on the market.
| 37 |
Our Platform Technology
We will continue to evolve and improve bfLEAP™, either in-house or with development partners like JHU-APL. The bfLEAP™ platform is based on an exclusive, worldwide license granted by JHU.
We plan to leverage our proprietary AI/ML platform developed over several years at one of the top innovation institutions in the world which has already been successfully applied in multiple sectors. In terms of underlying intellectual property, we have secured a worldwide exclusive license from JHU-APL for the technology - this license covers 3 issued patents, as well as 1 new provisional patent application, non-patent rights to proprietary libraries of algorithms and other trade secrets, and also includes modifications and improvements. In addition, we have a unique business model designed to reduce risk and increase the frequency of cash flow.
The Company has recently licensed new technology from JHU-APL to evolve the bfLEAP platform to bfLEAP 2.0. This new and improved platform will enable more robust analysis of data with faster and higher precision prediction of the most important variables for identifying patient response to a drug.
Going forward, the Company will continue to evolve the platform and either develop or acquire new capabilities and technologies. These development efforts may be in house or in collaboration with an existing or new technology partners. The Company plans on hiring talent in data science and software development to bolster its in house capabilities.
Summary for CATIE Schizophrenia Case Study
The Company worked with the Lieber Institute for Brain Development to analyze data from the landmark CATIE trials. The CATIE trials were the largest trials ever conducted for anti-psychotic medications. BullFrog analyzed CATIE data from ~200 schizophrenia patients, with a library of almost 1 million genetic data points for each patient, more than 200 non-genetic attributes per patient, and 4 different medications used in the trial. For each of the four medications used, bfLEAP™ analysis revealed new, previously unknown relationships between individual genetic variants and negative patient symptoms. The genetic loci identified represent potential druggable targets, as well as potential stratifying criteria for future clinical trials in schizophrenia.
We performed another analysis on the data using our new advanced clustering algorithms bfLEAP 2.0 but focused on one particular drug named Olanzapine. Our bfLEAP™ 2.0 analytical results identified previously unknown, multi-dimensional associations among newly identified genetic variants, drug clearance, clinical trial sites, and clinical outcome variables in schizophrenia patients.

| 38 |
FIGURE 1 - bfLEAP™ Analytical Map
Each green node represents a different sampling of the data, and arrows point to attributes (blue nodes) which were found to be key indicators according to that sampling. Attribute importance is determined by how many samplings identify that attribute as an indicator (i.e., number of incoming arrows to each blue node).
| 39 |
Identification of clustered multi-variate associations (e.g., novel genetic variants, drug clearance, substance abuse) could help us 1) identify novel drug targets, 2) predict which patients are most likely to respond, and 3) identify modifiable factors that could contribute to better outcomes.
Summary for Cardiovascular Case Study
The Company worked with an international collaborator in cardiovascular devices to analyze data from an ongoing clinical trial for a new device. BullFrog analyzed data from ~55 patients, with a library of almost 15,000 unique attributes of data for each patient. The data also included adverse events, and key demographic information. For this collaborator, bfLEAP™ analysis was able to provide ground truth for the company - confirming multiple correlations and non-correlations within the data. In terms of actionable output, the analytical results confirmed at least two demographic co-variates for the ongoing trial, and also provided a starting point for deeper physiological and molecular studies.
Our Supply Chain and Customer Base
We have launched our businesses using funds from our initial public offering and through our partnerships and relationships. We have a strategic relationship with FSHD Society, a leading non-governmental organization, for AI/ML analysis of clinical trial data for patients with a rare neuromuscular disorder. We also have several other developing strategic relationships in the project design phase. The Company has executed a joint development deal for a biologics discovery phase opportunity that is directed toward targeted cancer therapeutics. The Company has also obtained exclusive worldwide rights to a Phase 2 ready glioblastoma drug and a discovery phase hepatocellular carcinoma drug from universities. Since we intend to conduct late-stage clinical trials with partners on rescued therapeutic assets, there will be a requirement of drug product or other significant services to plan and execute our clinical development programs. The success of our partnered clinical development programs will require adequate availability of raw materials and/or drug product for our R&D and clinical trials, and, in some cases, may also require establishment of third-party arrangements to obtain finished drug product that is manufactured appropriately under industry-standard guidelines, and packaged for clinical use or sale. Since we are a digital biopharmaceutical company, our clinical development programs will also require, in some cases, establishment of third-party relationships for execution and completion of clinical trials.
Our Market Opportunity
One aim of our business is to “rescue” drugs that have failed in phase 3 clinical trials by using our technology to analyze all available data with the goal of designing a precision medicine clinical trial that will have a better chance of being successful. The graphic below illustrates the estimated market opportunity for these failed drugs. The top arrow shows the number of failed phase 3 trials for several disease categories over a 5-year period. The arrows below provide our assumptions for narrowing or discounting certain parameters associated with the market size calculation. The final arrow shows the math behind the $47.1B. To date, we have not penetrated the failed drug market, however; we are actively searching for failed drug opportunities.
| 40 |
Identification of candidates with potential for rescue may be challenging and require significant resources, and once these assets are identified the Company may find it challenging to license them under favorable terms in order to create value for shareholders. Subsequent development of these assets for clinical testing may require significant effort and resources. Ultimately, these assets must undergo rigorous clinical testing and approval by FDA or comparable regulatory authorities in other countries in order to be marketed. A key part of our strategy is to partner our R&D programs. In addition, we do not intend on commercializing drugs and instead will seek to divest each drug asset to a company that will commercialize the drug. The Company may receive future royalties in come transactions.
The following graphic illustrates the global revenue forecast for applying AI in the pharmaceutical industry, as well as the increase in anticipated market spend and annual growth rate for AI solutions per certain application areas.
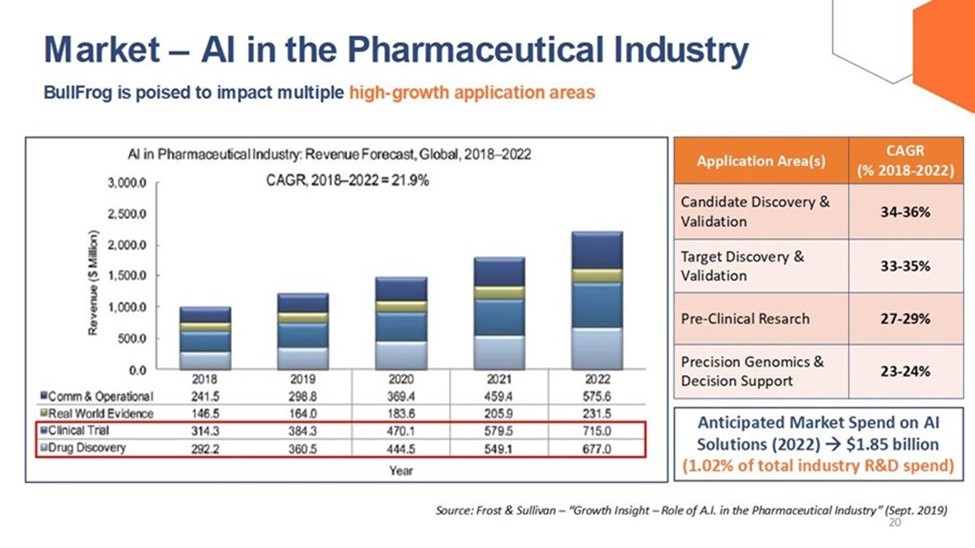
| 41 |
Intellectual Property
Patents
We have exclusive worldwide rights to the following patents related to our intellectual property:
Johns Hopkins University Licensed Intellectual Property:
| Title | Serial Number | File Date | Application Type | Country | Status | Patent Number | Expiration Date | Assignee | ||||||||
| An Improved Formulation of Mebendazole and Drug Combination to Improve Anti-cancer Activity | 62/112,706 | 06 Feb 2015 | Provisional | US | Expired | The Johns Hopkins University | ||||||||||
| An Improved Formulation of Mebendazole and Drug Combination to Improve Anti-cancer Activity | PCT/US2016/016968 | 08 Feb 2016 | PCT | PCT - Parent | Expired | 11 Aug 2016 | The Johns Hopkins University | |||||||||
| MEBENDAZOLE POLYMORPH FOR TREATMENT AND PREVENTION OF TUMORS | 15/548,959 | 04 Aug 2017 | PCT | US | GRANTED | 11,110,079 | 08 Feb 2036 | The Johns Hopkins University | ||||||||
| Mebendazole Polymorph For Treatment And Prevention Of Tumors | 16747414.7 | 08 Feb 2016 | PCT | EPO | GRANTED | Pending | 08 Feb 2036 | The Johns Hopkins University | ||||||||
| MEBENDAZOLE POLYMORPH FOR TREATMENT AND PREVENTION OF TUMORS | 253854 | 08 Feb 2016 | PCT | Israel | GRANTED | 253854 | 08 Feb 2036 | The Johns Hopkins University | ||||||||
| An Improved Formulation of Mebendazole and Drug Combination to Improve Anti-cancer Activity | 2016800144274 | 08 Feb 2016 | PCT | China | GRANTED | 1ZL20168-0014427.4 | 08 Feb 2036 | The Johns Hopkins University | ||||||||
| An Improved Formulation of Mebendazole and Drug Combination to Improve Anti-cancer Activity | 201717028684 | 08 Feb 2016 | PCT | India | GRANTED | 352734 | 08 Feb 2036 | The Johns Hopkins University | ||||||||
| Mebendazole Polymorph For Treatment And Prevention Of Tumors | 2017-541687 | 08 Feb 2016 | PCT | Japan | GRANTED | 6796586 | 08 Feb 2036 | The Johns Hopkins University | ||||||||
| CONTINUATION: Mebendazole Polymorph For Treatment And Prevention Of Tumors | 17/402,131 | 13 Aug 2021 | CON | United States | PENDING | The Johns Hopkins University |
| 42 |
George Washington University Licensed Intellectual Property:
The provisional patent numbers 63/113,745 and 63/147,141 were both converted into a single PCT application (PCT/US2021/059245) with an expiration date of November 12, 2041, as shown in table below.
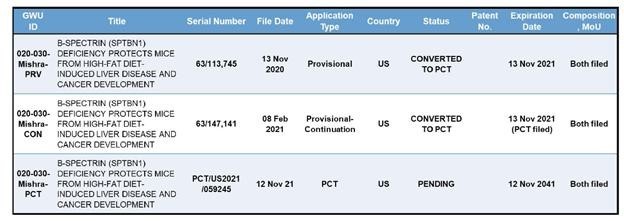
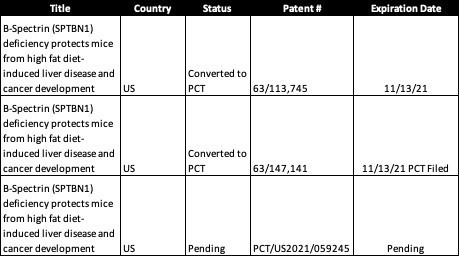
John Hopkins University Applied Physics Lab Licensed Intellectual Property:
| Title | Serial Number | File Date | Country | Status | Expiration Date | Assignee | ||||||
| Apparatus and Method for Distributed Graph Processing | U.S. Patent 10,146,801 | 7/13/2015 | US | Granted | 3/2/2037 | The Johns Hopkins University | ||||||
| Method and Apparatus for Analysis and Classification of High Dimensional Data Sets | U.S. Patent 10,936,965 | 10/5/2017 | US | Granted | 9/25/2038 | The Johns Hopkins University | ||||||
| Generalized Low Entropy Mixture Model | U.S. Patent 10,839,256 | 4/2/2018 | US | Granted | 12/15/2038 | The Johns Hopkins University |
Licenses
We hold the following licenses related to our intellectual property:
| Licensor | Licensee | Description of Rights Granted | ||
| Johns Hopkins University Applied Physics Lab | BullFrog AI, Inc. | Worldwide, exclusive rights for therapeutics development and analytical services | ||
| George Washington University | BullFrog AI Holdings | Worldwide, exclusive rights for therapeutics development | ||
| Johns Hopkins University | BullFrog AI Holdings | Worldwide, exclusive rights for therapeutics development |
| 43 |
On February 7, 2018, we entered into a License Agreement (the “License Agreement”) with JHU-APL, a Maryland limited liability company (“JHU”). Pursuant to the License Agreement, JHU-APL granted the Company exclusive rights to intellectual property of JHU related to analytical services for applications in biological and chemical derived pharmaceutical therapeutics. The License Agreement provides for the grant of an exclusive, worldwide, royalty-bearing license by JHU to the Company, with the right to sublicense, in order to conduct research using the patent rights and know-how and to develop and commercialize products in the field using the patent rights and know-how. In consideration of the rights granted to the Company under the License Agreement, the Company granted JHU received a warrant equal to five (5%) percent of the then fully diluted equity base of the Company, which was diluted following our public offering. Under the terms of the License Agreement, the Company is required to use commercially reasonable efforts to meet certain development milestones and minimum net sales milestones, and JHU will be entitled to eight (8%) percent of net sales for the services provided by the Company in which the JHU license was utilized, as well as fifty (50%) percent of all sublicense revenues received by the Company. In addition, the Company is required to pay JHU an annual maintenance fee of $1,500. The Company is also obligated to make minimum annual payments. These minimum annual payments to JHU were amended on September 3, 2020 to $20,000 in calendar year 2022, $80,000 in calendar year 2023, $300,000 in calendar year 2024, and $300,000 in calendar year 2025 and each year thereafter, which may be offset against royalties paid by the Company for the year in which the minimum annual royalty becomes due.
The License Agreement will, unless sooner terminated, continue in each country until the date of expiration of the last to expire patent included within the patent rights in that country, or if no patents issue, then for 10 years. The License Agreement may be terminated by the Company upon 60 days’ written notice in its discretion. The License Agreement may also be terminated by JHU if the Company is in material breach of the License Agreement and fails to cure such breach within a 60-day cure period commencing upon notice. A material breach by the Company may include a delinquency with respect to payment or the failure by the Company to timely achieve a specified milestone.
We also have exclusive, worldwide licenses to other intellectual property from JHU that are being held as trade secrets related to our algorithm libraries, pattern recognition, shallow-and-wide data sets, and time series correlation. We anticipate that new intellectual property (patents, copyrights, trademarks, trade secrets, etc.) will be generated through the course of executing our strategic development projects, and also through the course of improving, modifying, and scaling our bfLEAP™ platform. In October 2021, we amended the agreement with JHU-APL to include additional advanced AI technology. Currently, the latest patent grant date was in March 2021.
On July 8, 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from JHU-APL for the additional technology (the “2022 License Agreement”). This license provides additional intellectual property rights including patents, copyrights and knowhow to be utilized under the Company’s bfLEAP™ analytical AI/ML platform. Under the terms of the 2022 License Agreement, JHU will be entitled to eight (8%) percent of net sales for the services provided by the Company to other parties and 3% for internally development drug projects in which the JHU license is utilized. The 2022 License Agreement also contains tiered sub licensing fees that start at 50% and reduce to 25% based on revenues. In addition, the Company is required to pay JHU an annual maintenance fee of $1,500. Minimum annual payments are set to be $30,000 for 2022, $80,000 for 2023, and $300,000 for 2024 and beyond, all of which are creditable by royalties. The financial terms of the new license agreement replace the original terms and are not duplicative.
George Washington University - Beta2-spectrin siRNA License
On January 14, 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from GWU for rights to use siRNA targeting Beta2-spectrin in the treatment of human diseases, including HCC. The license covers methods claimed in three US and worldwide patent applications, and also includes use of this approach for treatment of obesity, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis. This program is currently in the preclinical stage of development. The Company has not yet initiated development activities or IND-enabling studies on this asset; however, the plan is to conduct this work over the next 24 months. All R&D to date on this candidate has been conducted by the licensor of the technology, George Washington University. The term of the agreement began on January 14, 2022 and ends on the expiration date of the last patent to expire or 10 years after the first sale of a licensed product if no patents have issued. The license can be terminated by the licensee upon 60 days’ written notice, or by the licensor if the Company is more than 30 days late in paying amounts owed to the licensor and does not make payment upon demand, or in the event of any material breach of the license that is not cured within 45 days.
Non-alcoholic fatty liver disease (NAFLD) is a condition in which excess lipids, or fat, build up in the liver. This condition, which is more common in people who have obesity and related metabolic diseases including type 2 diabetes, affects as many as 24% of adults in the US and is associated with risk of progression to more serious conditions, including non-alcoholic steatohepatitis (NASH), with associated liver inflammation and fibrosis, and hepatocellular carcinoma (HCC). Evidence in animal models of obesity suggest that a protein called β2-spectrin may play a key role in lipid accumulation, tissue fibrosis, and liver damage, and targeting expression or activity of this protein may be a useful approach in treating NASH and liver cancer (Rao et al., 2021).
| 44 |
In consideration of the rights granted to the Company under the license agreement, GWU received a $20,000 License Initiation Fee. Under the terms of the License Agreement, GWU will be entitled to a three percent (3%) royalty on net sales subject to quarterly minimums once the first sale has occurred subsequent to regulatory approval, as well sublicense or assignment fees in the event the Company sublicenses or assigns their rights to use the technology. The Company will also reimburse GWU for previously incurred and ongoing patent costs. The Sublicense and Assignment fee amounts decline as the Company advances the clinical development of the licensed technology. The license agreement also contains milestone payments for clinical development through the approval of an NDA and commercialization.
Aggregate payments made to GWU to date include the $20,000 License Initiation Fee and an additional $6,550 to reimburse the licensor for past patent costs. Aggregate future milestone costs could reach $860,000 if the drug successfully completes clinical trials and is the subject of a New Drug Application (NDA) to the U.S. FDA. Future milestones on sales revenue are limited to $1M on the first $20M in net sales.
Johns Hopkins University - Mebendazole License
On February 22, 2022, the Company entered into an exclusive, worldwide, royalty-bearing license from JHU for the use of an improved formulation of Mebendazole for the treatment of any human cancer or neoplastic disease. This formulation shows potent activity in animal models of different types of cancer, and has been evaluated in a Phase I clinical trial in patients with high-grade glioma (NCT01729260). The trial, an open-label dose-escalation study, assessed the safety of the improved formulation with adjuvant temozolomide in 24 patients with newly diagnosed gliomas. Investigators observed no dose-limiting toxicity in patients receiving all but the highest tested dose (200mg/kg/day). Four of the 15 patients receiving the maximum tested dose of 200mg/kg/day experienced dose-limiting toxicity, all of which were reversed by decreasing or eliminating the dose given. There were no serious adverse events attributed to mebendazole at any dose during the trial. The Company is currently formulating a strategy to conduct additional clinical trials with this asset to enable evaluation of safety in humans.
The license covers six (6) issued patents and one (1) pending application, with the term of the agreement beginning on February 22, 2022 and ending on the date of expiration of the last to expire patent. The license can be terminated by the licensee upon 90 days’ written notice, or by the licensor in the event of any material breach of the license that is not cured within 30 days. In consideration of the rights granted to the Company under the license agreement, JHU will receive a staggered Upfront License Fee of $250,000, with the first $50,000 payment due within 30 days of the effective date. The Company will also reimburse JHU for previously incurred and ongoing patent costs. Under the terms of the license agreement, JHU will be entitled to three- and one-half percent (3.5%) royalty on net sales by the Company. In addition, the Company is required to pay JHU minimum annual royalty payments of $5,000 for 2023, $10,000 for 2024, $20,000 for 2025, $30,000 for 2026 and $50,000 for 2027 and each year after until the first commercial sale after which the annual minimum royalty shall be $250,000. The license agreement also contains milestone payments for clinical development steps through the approval of an NDA and commercialization. Aggregate payments made to date include the initial $50,000 upfront fee and an additional $79,232.53 to reimburse the licensor for past patent costs. Aggregate future milestone costs could reach $1,500,000 if the drug successfully completes Phase II and III clinical trials and is approved for sale and marketing by the US FDA. Future milestones on sales revenue are $1M on the first $20M in sales revenue, $2M in the first year cumulative sales revenue exceeds $100M, $10M in the first year cumulative sales revenue exceeds $500M, and $20M in the first year cumulative sales revenue exceeds $1B.
JHU - Mebendazole Prodrug License
On October 13, 2022, the Company entered into an exclusive, worldwide, royalty-bearing license from JHU and the Institute of Organic Chemistry and Biochemistry (IOCB) of the Czech Academy of Sciences for rights to commercialize N-substituted prodrugs of mebendazole that demonstrate improved solubility and bioavailability. The license covers prodrug compositions and use for treating disease as claimed in multiple US and worldwide patent applications. The term of the agreement began on October 13, 2022and continues until the date of expiration of the last to expire patent, or for 20 years from the effective date of the agreement if no patents issue. The license can be terminated by the Company upon 90 days’ written notice, or by the licensor in the event of any material breach of the license that is not cured by the Company within 30 days.
| 45 |
In consideration for the rights granted to the Company under the License Agreement JHU and IOCB will receive a staggered upfront license fee of $100,000. The Company will also reimburse JHU and IOCB for previously incurred patent costs totaling $33,265 and will be responsible for reimbursing licensors for future patent costs. Under the terms of the License Agreement, the licensors will be entitled to a four percent (4%) royalty on net sales subject to annual minimums upon first commercial sale of a licensed product, as well sublicense or assignment fees in the event the Company sublicenses or assigns their rights to use the technology. The Sublicense fee amount declines as the Company advances the clinical development of the licensed technology. The Company is required to pay minimum annual royalties (MAR) beginning in year 4 of the agreement. The MAR for year 4 will be $5,000, increasing to $10,000 in year 5, $20,000 in year 6, $30,000 in year 7, and $50,000 in year 8 and subsequent years. The Company will be responsible for milestone payments for patent issuance of up to $50,000 and clinical development milestones up to and including approval of an NDA totaling up to $2.3M. The Company will be required to pay a commercial milestone of $1M once sales reach $20M in the US, $2M when sales in the US reach $100M, $10M when US sales reach $500M, and $20M when US sales exceed $1B.
Competition
The pharmaceutical and biotechnology industries are characterized by rapidly advancing technologies, intense competition, and a strong emphasis on proprietary products. The immuno-oncology, neuroscience, and rare disease segments of the industry in particular are highly competitive. While we believe that our technology, development experience and scientific knowledge provide competitive advantages, we face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical, and biotechnology companies, academic institutions and governmental agencies, and public and private research institutions.
Many of our competitors may have significantly greater financial resources, and expertise in research and development, manufacturing, preclinical studies, conducting clinical trials, obtaining regulatory approvals, and marketing approved medicines than we do. Mergers and acquisitions in the pharmaceutical, biotechnology, and diagnostic industries may result in even more resources being concentrated among a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and in establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to or necessary for our programs. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
The key competitive factors affecting the success of all of our product candidates, if approved, are likely to be their efficacy, safety, convenience, price, the effectiveness of companion diagnostics in guiding the use of related therapeutics, if any, the level of generic competition and the availability of reimbursement from government and other third-party payors.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize medicines that are safer, are more effective, have fewer or less severe side effects, are more convenient or are less expensive than any medicines we may develop. Our competitors also may obtain FDA or other regulatory approval for their medicines more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, our ability to compete may be affected in many cases by insurers or other third-party payors seeking to encourage the use of generic medicines. There are many generic medicines currently on the market for certain of the indications that we are pursuing, and additional generics are expected to become available over the coming years. If our therapeutic product candidates are approved, we expect that they will be priced at a significant premium over competitive generic medicines.
Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future. If the product candidates of our priority programs are approved for the indications for which we are currently planning clinical trials, they will compete with the drugs discussed below and will likely compete with other drugs currently in development.
bfLEAP
The analytics industry and application of AI in healthcare is growing rapidly. Competition exists along the entire continuum of the drug development process from discovery to commercialization and beyond. We believe the weakness of the industry is the quality of the data and we believe bfLEAP provides several competitive advantages, that will position the Company for success, First, bfLEAP is highly scalable and can process data from small to extremely large complex data sets without the need for additional code being developed. Second, it is adept at processing and analyzing incomplete data and making predictions that we do not believe other technologies are capable of doing. Finally, bfLEAP has the ability to extract the most important features for analysis out of extremely large complex data sets using unsupervised machine learning algorithms, thereby greatly simplifying complex problems. Since data quality is a problem that exists in the healthcare industry, we see these as major differentiators. The ability to make predictions, find relationships and patterns and anomalies in extremely large complex data sets has been demonstrated by the Applied Physics Lab in other applications and sectors. Finally, the algorithms used by bfLEAP are proprietary and protected, having been developed at Johns Hopkins University Applied Physics Lab. We believe most of the competitors rely on open source algorithms and we also believe that we have already demonstrated our superiority via the August 2021 publication in DeepAI.org.
| 46 |
Government Regulation
The FDA does not currently require approval of AI technologies used to aid in therapeutics, but that could change in the future. The FDA will regulate any clinical trials conducted by the Company.
Our clinical development programs will, in some cases, require regulatory review of preclinical and/or clinical data by the FDA or other governing agencies, and subsequent compliance with applicable federal, state, local, and foreign statutes and regulations. The results of the clinical trials that we conduct will be evaluated by the FDA and other regulatory bodies. The comments and approvals that are obtained are expected to lead to milestone payments under the collaborative agreement. Accordingly, our ability to navigate the regulatory process is extremely important to the success of the Company. We believe that we have a competitive advantage in this process due to primarily focusing on drug candidates that already have some level of success in clinical trials. Previous success of a particular candidate in trials combined with our precision medicine approach to clinical trial design using our bfLEAP platform, will de-risk the development process and improve the chances for success.
Government Regulation and Product Approval
Government authorities in the United States, at the federal, state and local level, and in other countries and jurisdictions extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, post-approval monitoring and reporting, and import and export of pharmaceutical products. The processes for obtaining regulatory approvals in the United States and in foreign countries and jurisdictions, along with subsequent compliance with applicable statutes and regulations and other regulatory authorities, require the expenditure of substantial time and financial resources.
FDA Approval Process
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The Federal Food, Drug, and Cosmetic Act (FD&C Act) and other federal and state statutes and regulations govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending new drug applications (NDAs), warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties and criminal prosecution.
Pharmaceutical product development for a new product or certain changes to an approved product in the U.S. typically involves preclinical laboratory and animal tests, the submission to FDA of an investigational new drug application (IND) which must become effective before clinical testing may commence, and adequate and well-controlled clinical trials to establish the safety and effectiveness of the drug for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease.
Preclinical tests include laboratory evaluation of product chemistry, formulation and toxicity, as well as animal trials to assess the characteristics and potential safety and efficacy of the product. The conduct of the preclinical tests must comply with federal regulations and requirements, including good laboratory practices. The results of preclinical testing are submitted to FDA as part of an IND along with other information, including information about product chemistry, manufacturing and controls, and a proposed clinical trial protocol. Long-term preclinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted. A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If FDA has neither commented on nor questioned the IND within this 30-day period, the clinical trial proposed in the IND may begin. Clinical trials involve the administration of the investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted: (i) in compliance with federal regulations; (ii) in compliance with good clinical practice, or GCP, an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators and monitors; as well as (iii) under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to FDA as part of the IND.
| 47 |
Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, but the phases may overlap. In Phase 1, the initial introduction of the drug into healthy human subjects or patients, the drug is tested to assess metabolism, pharmacokinetics, pharmacological actions, side effects associated with increasing doses, and, if possible, early evidence of effectiveness. Phase 2 usually involves trials in a limited patient population to determine the effectiveness of the drug for a particular indication, dosage tolerance and optimum dosage, and to identify common adverse effects and safety risks. If a drug demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 trials are undertaken to obtain the additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit FDA to evaluate the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the drug. In most cases, FDA requires two adequate and well-controlled Phase 3 clinical trials to demonstrate the efficacy of the drug. A single Phase 3 trial with other confirmatory evidence may be sufficient in rare instances, such as where the study is a large multicenter trial demonstrating internal consistency and a statistically very persuasive finding of a clinically meaningful effect on mortality, irreversible morbidity or prevention of a disease with a potentially serious outcome and confirmation of the result in a second trial would be practically or ethically impossible.
After completion of the required clinical testing, an NDA is prepared and submitted to FDA. FDA approval of the NDA is required before marketing of the product may begin in the U.S. The NDA must include the results of all preclinical, clinical and other testing and a compilation of data relating to the product’s pharmacology, chemistry, manufacture and controls. The cost of preparing and submitting an NDA is substantial. The submission of most NDAs is additionally subject to a substantial application user fee, and the applicant under an approved NDA is also subject to an annual program fee for each prescription product. These fees are typically increased annually. Sponsors of applications for drugs granted Orphan Drug Designation are exempt from these user fees.
FDA may also refer applications for novel drug products, or drug products that present difficult questions of safety or efficacy, to an outside advisory committee - typically a panel that includes clinicians and other experts - for review, evaluation and a recommendation as to whether the application should be approved. FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations.
Before approving an NDA, FDA will typically inspect one or more clinical sites to assure compliance with GCP. Additionally, FDA will inspect the facility or the facilities at which the drug is manufactured. FDA will not approve the product unless compliance with current good manufacturing practices (cGMPs) is satisfactory and the NDA contains data that provide substantial evidence that the drug is safe and effective in the indication studied.
Fast Track Designation
FDA is required to facilitate the development, and expedite the review, of drugs that are intended for the treatment of a serious or life-threatening disease or condition for which there is no effective treatment and which demonstrate the potential to address unmet medical needs for the condition. Under the Fast Track program, the sponsor of a new drug candidate may request that FDA designate the drug candidate for a specific indication as a Fast Track drug concurrent with, or after, the filing of the IND for the drug candidate. FDA must determine if the drug candidate qualifies for Fast Track Designation within 60 days of receipt of the sponsor’s request.
If a submission is granted Fast Track Designation, the sponsor may engage in more frequent interactions with FDA, and FDA may review sections of the NDA before the application is complete. This rolling review is available if the applicant provides, and FDA approves, a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, FDA’s time period goal for reviewing an application does not begin until the last section of the NDA is submitted. While we may seek Fast Track Designation, there is no guarantee that we will be successful in obtaining any such designation. Even if we do obtain such designation, we may not experience a faster development process, review or approval compared to conventional FDA procedures. A Fast Track Designation does not ensure that the product candidate will receive marketing approval or that approval will be granted within any particular timeframe. Additionally, Fast Track Designation may be withdrawn by FDA if FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Post-Approval Requirements
Once an NDA is approved, a product will be subject to certain post-approval requirements. For instance, FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet. Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved labeling.
Adverse event reporting and submission of periodic reports are required following FDA approval of an NDA. FDA also may require post-marketing testing, known as Phase 4 testing, REMS and surveillance to monitor the effects of an approved product, or FDA may place conditions on an approval that could restrict the distribution or use of the product. In addition, quality control, drug manufacture, packaging and labeling procedures must continue to conform to cGMPs after approval. Drug manufacturers and certain of their subcontractors are required to register their establishments with FDA and certain state agencies. Registration with FDA subjects entities to periodic unannounced inspections by FDA, during which the Agency inspects manufacturing facilities to assess compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money and effort in the areas of production and quality-control to maintain compliance with cGMPs. Regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered.
| 48 |
Generic Competition
In seeking approval for a drug through an NDA, applicants are required to list with the FDA each patent whose claims cover the applicant’s product. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential generic competitors in support of approval of an abbreviated new drug application (ANDA). An ANDA provides for marketing of a drug product that has the same active ingredients in the same strengths and dosage form as the listed drug and has been shown through bioequivalence testing to be therapeutically equivalent to the listed drug. Other than the requirement for bioequivalence testing, ANDA applicants are not required to conduct, or submit results of, preclinical or clinical tests to prove the safety or effectiveness of their drug product. Drugs approved in this way are commonly referred to as “generic equivalents” to the listed drug and can often be substituted by pharmacists under prescriptions written for the original listed drug.
The ANDA applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA’s Orange Book. Specifically, the applicant must certify that (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product (a Paragraph IV certification). The ANDA applicant may also elect to submit a section viii statement certifying that its proposed ANDA label does not contain (or carve out) any language regarding the patented method-of-use rather than certify to a listed method-of-use patent. If the applicant does not challenge the listed patents or certifies that the listed patents will not be infringed by the new product, the ANDA application will not be approved until all the listed patents claiming the referenced product have expired. If the ANDA applicant has provided a Paragraph IV certification, the NDA and patent holders may then initiate a patent infringement lawsuit in response. The filing of a patent infringement lawsuit within 45 days of the receipt of a such certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit, or a decision in the infringement case that is favorable to the ANDA applicant.
Exclusivity
Upon NDA approval of a new chemical entity (NCE) that drug receives five years of marketing exclusivity during which FDA cannot receive any ANDA seeking approval of a generic version of that drug. An ANDA may be submitted one year before NCE exclusivity expires if a Paragraph IV certification is filed. If there is no listed patent in the Orange Book, there may not be a Paragraph IV certification, and, thus, no ANDA may be filed before the expiration of the exclusivity period. Certain changes to a drug, such as the addition of a new indication to the package insert, can be the subject of a three-year period of exclusivity if the application contains reports of new clinical investigations (other than bioavailability studies) conducted or sponsored by the sponsor that were essential to approval of the application. FDA cannot approve an ANDA for a generic drug that includes the change during the period of exclusivity.
Patent Term Extension
After NDA approval, owners of relevant drug patents may apply for up to a five-year patent extension. The allowable patent term extension is calculated as half of the drug’s testing phase (the time between IND application and NDA submission) and all of the review phase (the time between NDA submission and approval up to a maximum of five years). The time can be shortened if FDA determines that the applicant did not pursue approval with due diligence. The total patent term after the extension may not exceed 14 years, and only one patent can be extended. For patents that might expire during the application phase, the patent owner may request an interim patent extension. An interim patent extension increases the patent term by one year and may be renewed up to four times. For each interim patent extension granted, the post-approval patent extension is reduced by one year. The director of the United States Patent and Trademark Office must determine that approval of the drug covered by the patent for which a patent extension is being sought is likely. Interim patent extensions are not available for a drug for which an NDA has not been submitted.
Other Healthcare Laws
In the United States, biotechnology company activities are subject to regulation by various federal, state and local authorities in addition to the FDA, including but not limited to, the Centers for Medicare & Medicaid Services (CMS), other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General and the Office for Civil Rights), the U.S. Department of Justice (DOJ) and individual U.S. Attorney offices within the DOJ, and state and local governments. For example, research, sales, marketing and scientific/educational grant programs have to comply with the anti-fraud and abuse provisions of the Social Security Act, the federal false claims laws, the privacy and security provisions of the Health Insurance Portability and Accountability Act (HIPAA) and similar state laws, each as amended, as applicable.
| 49 |
Also, many states have similar fraud and abuse statutes or regulations that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
Data privacy and security regulations by both the federal government and the states in which business is conducted may also be applicable. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, imposes requirements relating to the privacy, security and transmission of individually identifiable health information. HIPAA requires covered entities to limit the use and disclosure of protected health information to specifically authorized situations and requires covered entities to implement security measures to protect health information that they maintain in electronic form. Among other things, HITECH made HIPAA’s security standards directly applicable to business associates, independent contractors or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Insurance Coverage and Reimbursement
Significant uncertainty exists as to the insurance coverage and reimbursement status of any products for which we may obtain regulatory approval. In the United States, sales of any product candidates for which regulatory approval for commercial sale is obtained will depend in part on the availability of coverage and adequate reimbursement from third-party payors. Third-party payors include government authorities and health programs in the United States such as Medicare and Medicaid, managed care providers, private health insurers and other organizations. These third-party payors are increasingly reducing reimbursements for medical products and services. The process for determining whether a payor will provide coverage for a drug product may be separate from the process for setting the reimbursement rate that the payor will pay for the drug product. Third-party payors may limit coverage to specific drug products on an approved list, or formulary, which might not include all of FDA-approved drugs for a particular indication. A payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Further, coverage and reimbursement for drug products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance.
| 50 |
Recent Developments
Since our initial public offering, which took place in February 2023, the following material developments occurred in our business.
APL
On May 31, 2023, the Company and JHU-APL entered into Amendment No. 1 of the July 8, 2022 License Agreement whereby the Company gained access to certain improvements, including additional patents and know-how, in exchange for a series of payments totaling $275,000. The Company paid the first of these payments in the amount of $75,000 due in July 2023 and will be followed by payments in the amounts of $75,000, $75,000 and $50,000 in years 2025, 2026 and 2027, respectively. The amendment also reduced the 2023 minimum annual royalty payment to $60,000. All other financial terms of the License Agreement remain the same.
LIBD
On September 8, 2023, the Company entered a data use and technology partnership agreement (the “Partnership Agreement”) with the Lieber Institute for Brain Development (LIBD). The Partnership Agreement covers the right of BullFrog AI to leverage its bfLEAP™ platform to mine LIBD’s comprehensive brain data, including transcriptomic, genomic, DNA methylation, cell-line, clinical, and imaging data to identify previously unrecognized relationships. The goal of the partnership is to identify previously unrecognized relationships between genes and pathways in the brain and the development of neurologic and psychiatric disorders, thereby facilitating the development of more effective treatments for diseases of the human brain. The collaboration will proceed in two stages, with the first involving unsupervised construction of graphical models to reveal relationships between brain diseases and genomic/biologic attributes, with the goal of identifying new biomarkers and drug targets across disorders. The second stage will involve creating disease-specific models that will enable identification of genes and pathways within these respective disorders. The Partnership Agreement has a one-year term of data exclusivity to complete the first stages of analyses, with a two-year extension option as performance milestones are met.,
As contemplated in the Partnership Agreement, on October 16, 2023, the Company and LIBD entered into a commercial agreement (the “Commercial Agreement”) that sets forth the key terms for commercialization of products and services developed under the Partnership Agreement. Pursuant to the Commercial Agreement, LIBD granted the Company a worldwide, royalty-bearing exclusive license so long as the Company receives net sales or income from the licensing of “Licensed Products” (as defined in the Commercial Agreement) in the application of machine learning and/or artificial intelligence for research and development in drug development, and specifically includes therapeutic products, patient selection strategies, and target identification, but excludes diagnostics and incidental uses of machine learning and/or artificial intelligence on data derived from research. Generally, “Licensed products” are any product or service which incorporates, results from, or is derived from LIBD’s Data (meaning finished brain-related data, including but not limited to DNA methylation, RNAseq, genomic, DNA methylation, cell-line, clinical, and imaging data, and the specified data set forth in the Partnership Agreement) and that the Company or its affiliate develops during the term of the Partnership Agreement, and any improvements thereof after the term of the Partnership Agreement, and all Licensed Products or services derived therefrom by the Company or its affiliates. Licensed Products may include, but are not limited to, biomarker and target identification, target validation, mapping unmet needs, identifying genetic risk factors and predictive modeling.
The Company was also granted the right to sublicense, to use the deliverables under the Partnership Agreement, and LIBD’s intellectual property rights in the data, to (i) use, sell, distribute for sale, have distributed for sale, offer for sale, have sold, import and have imported Licensed Products and (ii) to develop, have developed, make, have made Licensed Products that are derived from Licensed Products developed during the term of the Partnership Agreement, and any improvements made following the term. The Company is prohibited from sublicensing LIBD Data. The Company shall pay LIBD a royalty based on net sales of all Licensed Products sold by the Company and/or its affiliates.
The Commercial Agreement, generally, may be terminated at any time by either the Company or LIBD if either party defaults or breaches any material term of the agreement or files for protection under bankruptcy laws, makes an assignment for the benefit of creditors, appoints or suffers appointment of a receiver, trustee, or similar agent over its property.
| 51 |
Prodrug
On September 26, 2023, the Company announced positive data in a preclinical study investigating the anti-cancer activity of a novel prodrug of mebendazole for the treatment of glioblastoma. The study assessed the relative efficacy of BF-222, a novel formulation of mebendazole that has been evaluated in clinical trials, and BF-223, a novel prodrug of mebendazole with improved solubility and bioavailability relative to BF-222, compared with placebo in mice that had been implanted with tumor cells as a model for human glioblastoma. Animals treated with BF-223 had an average survival time of 27.9 days compared with 27.3 days for mice treated with BF-222 and 23.4 days for mice given placebo. Mice treated with BF-223 were administered 80% of the dose that mice treated with BF-222 received, and improved outcomes for both treatment groups were statistically significant compared to placebo. In addition, animals treated with equivalent doses of BF-222 and BF-223 showed comparable and significant reduction in tumor growth compared to control animals during the study.
Employees
As of January 1, 2024, the Company has 8 full-time employees and consultants, including its Chief Executive Officer Vininder Singh and its Chief Financial Officer, Dane Saglio and 7 part-time employees, advisors, and consultants. None of these employees are covered by a collective bargaining agreement, and we believe our relationship with our employees is good. We also engage consultants on an as-needed basis to supplement existing staff.
Properties
Currently, the Company does not own any real property. All of the Company’s employees work virtually.
Legal Proceedings
The Company is not a party to any legal proceedings.
Corporate Information
BullFrog Holdings AI, Inc. was incorporated in the State of Nevada on February 6, 2020. Our principal business address is 325 Ellington Blvd., Unit 317, Gaithersburg, MD 20878. Our website address is www.bullfrogai.com. The references to our website in this prospectus are inactive textual references only. The information on our website is neither incorporated by reference into this prospectus nor intended to be used in connection with this Offering. All of our operations are currently conducted through BullFrog AI Holdings, Inc.
Available Information
Reports we file with the Securities and Exchange Commission (SEC) pursuant to the Exchange Act, including annual and quarterly reports, and other reports we file, can be inspected and copied at the public reference facilities maintained by the SEC at 100 F Street NE, Washington, D.C. 20549.
| 52 |
MANAGEMENT AND BOARD OF DIRECTORS
Executive Officers and Directors
The following table sets forth the name, age and position of each of our executive officers, key employees and directors.
| Name | Age | Position(s) | ||
| Executive Officers: | ||||
| Vin Singh | 54 | Chief Executive Officer and Director | ||
| Dane Saglio | 66 | Chief Financial Officer | ||
| Non-Executive Directors: | ||||
| Don Elsey | 70 | Director and Chair Audit Committee | ||
| William Enright | 60 | Director and Chair of Compensation Committee | ||
| Jason Hanson | 54 | Director and Chair of Nominating and Corporate Governance Committee |
Vininder (Vin) Singh is the Founder, Chairman, and CEO of BullFrog AI Holdings, Inc. since its inception in August 2017. Over the past five years, he has built the Company from scratch and during that time he led strategy, built a highly experienced team of leaders, spear headed the acquisition and development of BullFrog’s core AI technology and drug assets, secured the first revenue, and raised approximately $2M in financing. In February of 2020, he formed BullFrog AI Holdings, Inc. and BullFrog AI Inc. became a wholly owned subsidiary designated as the holder of core intellectual property. Vin is a serial entrepreneur and experienced executive with 25 years of experience in the life sciences and biotechnology industries. He has extensive start-up experience having founded and built several pioneering investor backed companies including BullFrog AI, which uses machine learning/AI to enable drug development, Next Healthcare Inc., a personalized diagnostics and adult cell banking service, and MaxCyte Inc. (MXCT), a cell therapy company. He was also an executive at GlobalStem Inc. and ThermoFisher Scientific, leading their global cell therapy services business. Vin has a BS in Electrical Engineering from Rutgers University, an MS in Biomedical Engineering from Rensselaer Polytechnic Institute, and an MBA from Johns Hopkins University. We believe that Mr. Singh is qualified to serve as a member of our board of directors due to the perspective and experience that he brings as our Founder and Chief Executive Officer, his extensive experience in the science and biotechnology industries and in the management of startup companies.
Dane Saglio joined BullFrog Holdings AI, Inc. as Chief Financial Officer in September 2021. Mr. Saglio brings more than 40 years of financial management experience in both public and private companies across a number of business sectors. Previously, Mr. Saglio has served as CFO at Seneca Biopharma, RegeneRx Biopharmaceuticals since 2011, New Generation Biofuels 2010 until 2011, and EntreMed from 2000 until 2008, all public companies in the biotechnology arena. Prior to joining the Company, Mr. Saglio was the CFO of Seneca Biopharma, initially as a consultant in August 2019 and then as an employee in April 2020 until the Company merged with Leading Bio Sciences, forming Palisades Bio, Inc. in April 2021. He previously served as CFO at Celios Corporation from October 2017 until July 2019 and Helomics Corporation, a personalized medicine company in cancer from October 2014 through July 2017. He began his career at Informatics Corp, now Computer Associates International and then at Bressler & Reiner, a DC-based real estate developer and homebuilder. Dane has a BS from the University of Maryland is a licensed CPA in Maryland (inactive).
R. Don Elsey has been a director and chair of the Audit Committee of our board since February 14, 2023. Currently, Mr. Elsey is the Audit Chair of OpGen, Inc., a precision medicine company. Mr. Elsey was the CFO of Lyra until his retirement in December 2020. Previously, from February 2015 to February 2019, Mr. Elsey served as Chief Financial Officer at Senseonics, Inc., a medical device company. From May 2014 until February 2015, Mr. Elsey served as Chief Financial Officer of Regado Biosciences, Inc., a biopharmaceutical company. From December 2012 to February 2014, Mr. Elsey served as Chief Financial Officer of LifeCell Corporation, a privately held regenerative medicine company. Mr. Elsey holds a B.A. in economics and an M.B.A. in finance from Michigan State University. We believe that Mr. Esley is qualified to serve as a member of our board of directors because of his extensive professional experience in science and biotechnology companies.
William “Bill” Enright has been a director and chair of the Compensation Committee of our board since February 14, 2023. He is a seasoned biotech executive with more than thirty-four years of experience in building and financing both privately held and publicly held companies and He is currently the CEO and a Director of Barinthus Biotherapurtics plc (NASDAQ: BRNS), which he helped to take public in April 2021. Prior to Barinthus, Bill spent more than ten years at Altimmune (NASDAQ: ALT) as a Director, President & CEO, moving multiple programs into clinical testing, completing several acquisitions, and eventually taking the company public. Prior to joining Altimmune, Bill spent six years with GenVec, Inc. (acquired by Precigen) with increasing responsibilities, culminating as Head of Business Development. Bill brings a breadth of experiences in a variety of positions within the life science/biotech industry, including time as a consultant, a bench scientist and 12 years with Life Technologies, Inc. (acquired by Thermo-Fisher), working in various senior level licensing, business management, manufacturing and research roles. Bill received a Master of Arts in Molecular Biology from SUNY at Buffalo and a Master of Science in Business Management from Johns Hopkins University. We believe that Mr. Enright is qualified to serve as a member of our board of directors because of his extensive professional experience in life science/biotech companies and in the management of public companies.
| 53 |
Jason Hanson has served as a director and chair of the Nominating and Corporate Governance Committee since February 14, 2023. Mr. Hanson has served as Chief Executive Officer and as a Director of enGene Inc. since July 2018. He also served as President of enGene Inc. from July 2018 to December 2022. Mr. Hanson effectively re-launched enGene from a small private company working in the GI discovery space into a clinical stage gene therapy oncology company trading on Nasdaq, implementing a new scientific, technical and strategic vision for the Company. From August 2016 to November, 2017, Mr. Hanson served as President and Chief Executive Officer of Ohana Biosciences, a biotechnology company based in Cambridge, MA, and as member of the Ohana Board of Directors and consultant to Ohana from November 2017 to June 2018. Mr. Hanson previously served as Executive Vice President and Chief Strategy Officer for NuVasive, Inc. from November 2015 to August 2016. Mr. Hanson served as Corporate Vice President of General Electric Company and member of the senior executive team of GE Healthcare, a global pharmaceutical, medical device and healthcare services business from May 2014 to October 2015. In January 2013, Mr. Hanson served as Company Group Chairman and Executive Vice President of Valeant Pharmaceuticals International, Inc. (now Bausch Health Companies Inc.). Previously, he served in various roles at Medicis Pharmaceutical Corporation, including as Executive Vice President and Chief Operating Officer between July 2006 and December 2012. Mr. Hanson also served in numerous roles at GE Healthcare, including General Counsel roles, from April 1999 to July 2006. Mr. Hanson holds a B.S. from Cornell University and a J.D. from Duke University School of Law.
Corporate Governance
Role of Board of Directors in Risk Oversight Process
The board of directors has extensive involvement in the oversight of risk management related to us and our business and accomplishes this oversight through the regular reporting by the Audit Committee.
Director Independence
Messrs. Elsey, Enright and Hanson, three members of our Board of Directors, are independent using the definition of independence under Nasdaq Listing Rule 5605(a)(2) and the standards established by the SEC.
Committees of our Board
Audit Committee
Our audit committee consists of Don Elsey, William Enright and Jason Hanson, with Mr. Elsey serving as chair. Our board of directors has affirmatively determined that each meets the definition of “independent director” under the rules of The Nasdaq Capital Market, and that they meet the independence standards under Rule 10A-3. Each member of our audit committee meets the financial literacy requirements of Nasdaq rules. Our board of directors has adopted a written charter for the audit committee, which can be found on our website at https://ir.bullfrogai.com/corporate-governance/governance-documents.
The audit committee is appointed by the board of directors to assist the board of directors in its duty to oversee the Company’s accounting, financial reporting, and internal control functions and the audit of the Company’s financial statements. The role of the audit committee is to oversee management in the performance of its responsibility for the integrity of the Company’s accounting and financial reporting and its systems of internal controls, the performance and qualifications of the Company’s independent auditor, including the independent auditor’s independence, the performance of the Company’s internal audit function; and the Company’s compliance with legal and regulatory requirements. The Audit Committee met four times in 2023.
Compensation Committee
Our compensation committee consists of William Enright, Don Elsey and Jason Hanson, with Mr. Enright serving as chair. Our board of directors has adopted a written charter for the compensation committee, which can be found on our website at https://ir.bullfrogai.com/corporate-governance/governance-documents.
The compensation committee is responsible for reviewing and recommending, among other things:
| ● | the adequacy and form of compensation of the board; | |
| ● | the compensation of Chief Executive Officer, including base salary, incentive bonus, stock option and other grant, award and benefits upon hiring and on an annual basis; | |
| ● | the compensation of other senior management upon hiring and on an annual basis; and | |
| ● | the Company’s incentive compensation and other equity-based plans and recommending changes to such plans to our board of directors, when necessary. |
Nominating & Corporate Governance Committee
Our nominating and corporate governance committee consists of Jason Hanson, William Enright and Don Elsey, with Mr. Hanson serving as chair. Our board of directors has adopted a written charter for the nominating and corporate governance committee, which can be found on our website at https://ir.bullfrogai.com/corporate-governance/governance-documents.
The nominating committee is responsible for, among other things:
| ● | developing criteria for membership on the board of directors and committees; | |
| ● | identifying individuals qualified to become members of the board of directors; | |
| ● | recommending persons to be nominated for election as directors and to each committee of the board of directors; | |
| ● | annually reviewing our corporate governance guidelines; and | |
| ● | monitoring and evaluating the performance of the board of directors and leading the board in an annual self-assessment of its practices and effectiveness. |
Term of office
All directors hold office until the next annual meeting of the stockholders of the company and until their successors have been duly elected and qualified. Officers are elected by and serve at the discretion of our Board.
| 54 |
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, employees or persons performing similar functions. Our code of ethics can be found at https://ir.bullfrogai.com/corporate-governance/governance-documents.
Family Relationships
There are no family relationships among and between the issuer’s directors, officers, persons nominated or chosen by the issuer to become directors or officers, or beneficial owners of more than ten percent of any class of the issuer’s equity securities.
Involvement in Certain Legal Proceedings
From time to time, we may become involved in litigation relating to claims arising out of its operations in the normal course of business. Currently there are no legal proceedings, government actions, administrative actions, investigations or claims are currently pending against us or that involve the Company or any of its affiliates which, in the opinion of the management.
EXECUTIVE AND DIRECTOR COMPENSATION
Summary Compensation Table
The following table sets forth all compensation awarded or paid to our principal executive officer and our two other most highly compensated executive officers during the past two fiscal years.
Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | All Other Compensation ($) | Total Compensation ($) | |||||||||||||||||||||
| Vininder Singh | 2023 | $ | 707,666 | $ | - | $ | - | $ | - | $ | - | $ | 707,666 | |||||||||||||||
| Chief Executive Officer and Director | 2022 | $ | 179,000 | - | - | - | - | $ | 179,000 | |||||||||||||||||||
| Dane Saglio | 2023 | $ | 310,000 | $ | 50,000 | $ | - | $ | 147,000 | $ | - | $ | 507,000 | |||||||||||||||
| Chief Financial Officer | 2022 | $ | 30,000 | $ | - | $ | - | $ | - | $ | - | $ | 30,000 | |||||||||||||||
Employment Agreements
On May 16, 2022, we entered into an employment agreement with Vininder Singh, pursuant to which he will receive received an annual base salary of $400,000, which is subject to bi-annual review by the Company. Mr. Singh will also be eligible for an annual bonus based on the achievement of certain goals and performance criteria established by the Board. Mr. Singh’s target annual bonus for the fiscal years ended 2022 through 2025 will be a minimum of twenty (20%) percent of the current base salary, with a maximum payout of up to one-hundred (100%) percent based on target achievement. For 2023, the criteria to determine Mr. Singh’s bonus will include the following: (i) the Company achieves $500,000 in sales; (ii) the filing of an Investigational New Drug (IND) Application with the FDA for mebandazole; (iii) the Company enters into two (2) strategic partnerships; and (iv) the Company commences partner negotiations with a third party for HSV-1, bf-114 or bf-222. Mr. Singh will also be eligible to participate in the Company’s stock incentive plan, subject to Board approval. The agreement with Mr. Singh shall continue until either his resignation, termination for cause by the Company, or death or disability of Mr. Singh.
| 55 |
Outstanding Equity Awards at Fiscal Year-End
The following table summarizes the outstanding equity awards held by each named executive officer as of December 31, 2023. This table includes unexercised and unvested options and equity awards.
| Outstanding Equity Awards as of December 31, 2023 | ||||||||||||||||||||
| Option Awards | ||||||||||||||||||||
| Name | Date of Grant | Number of securities underlying unexercised options (#) exercisable | Number of securities underlying unexercised options (#) unexercisable | Equity incentive plan awards: Number of securities underlying unexercised unearned options (#) | Option exercise price ($) | Option expiration date | ||||||||||||||
| Dane Saglio | March 17, 2023 | 43,750 | 31,250 | - | 2.80 | March 17, 2033 | ||||||||||||||
Director Compensation
The following table summarizes the compensation paid to our executive and non-executive directors during the year ended December 31, 2023.
| Name | Fees Earned or Paid in Cash ($)(1) | Stock Awards ($) | Option Awards ($)(2) | All Other Compensation ($) | Total ($) | |||||||||||||||
| Vininder Singh(3) | - | - | - | - | - | |||||||||||||||
| Don Elsey | 39,375 | - | 197,200 | - | 236,575 | |||||||||||||||
| William Enright | 39,375 | - | 197,200 | - | 236,575 | |||||||||||||||
| Jason Hanson | 39,375 | - | 197,200 | - | 236,575 | |||||||||||||||
| (1) | Represents cash compensation for service as a director and as chair of a board committee during the fiscal year 2023. |
| (2) | Represents annual value of stock options issued during fiscal year 2023 under our 2022 Equity Incentive Plan. |
| (3) | Mr. Singh did not receive additional compensation for his service as a director of our Company during the fiscal year 2023. |
2022 Equity Incentive Plan
On November 30, 2022, our Board of Directors and shareholders adopted the 2022 Equity Incentive Plan (the “Plan”). Pursuant to the Plan, we are authorized to grant options and other equity awards to officers, directors, employees and consultants. The exercise price of each share of common stock purchasable under an award issued pursuant to the Plan, shall be determined by our compensation committee, in its sole discretion, at the time of grant, but shall not be less than 100% of the fair market of such share of common stock on the date the award is granted, subject to adjustment and conditions further described in the Plan. Our compensation committee shall also have sole authority to set the terms of all awards at the time of grant. As of December 31, 2023, there are 461,500 shares available under the Plan.
| 56 |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Other than compensation arrangements, including employment, with our directors and executive officers, and the other transactions discussed in the sections titled “Executive and Director Compensation”, the following is a description of each transaction that occurred in the last three fiscal years and each currently proposed transaction in which (i) the amounts involved exceeded or will exceed the lesser of (a) $120,000 or (b) 1% of the average of our total assets for the fiscal years ended December 31, 2023 or 2022; and (ii) any of our directors, executive officers or holders of more than 5% of our capital stock, or any member of the immediate family of, or person sharing the household with, the foregoing persons, had or will have a direct or indirect material interest.
On July 8, 2021, the Company entered into a Simple Agreement for Future Equity (SAFE), with a related party, Tivoli Trust, our second largest shareholder (the “Investor”), with an amount of $150,000, with 0% interest. Under the SAFE agreement, if there was an Equity Financing before the termination of this SAFE, on the initial closing of such Equity Financing, this SAFE would automatically convert into the number of shares of SAFE Preferred Stock equal to the Purchase Amount divided by the Conversion Price, which meant either: (1) the Safe Price (the price per share equal to the Post-Money Valuation Cap divided by the Company Capitalization) or (2) the Discount Price (the price per share of the Standard Preferred Stock sold in the Equity Financing multiplied by the Discount Rate), whichever calculation resulted in a greater number of shares of Safe Preferred Stock. This SAFE would automatically terminate (without relieving the Company of any obligations arising from a prior breach of or non-compliance with this SAFE) immediately following the earliest to occur of: (i) the issuance of Capital Stock to the Investor pursuant to the automatic conversion of this SAFE under agreement; or (ii) the payment, or setting aside for payment, of amounts due the Investor pursuant to the agreement. The SAFE was converted into 32,967 shares of common stock upon the Company’s initial public offering in February 2023.
On August 19, 2021, the company entered into a convertible loan agreement with a related party, with a principal balance of $99,900 at 9% interest. The noteholder had the right to convert the principal and interest into common shares of the Company. This loan included an original issuance discount of 5% and included 99,900 Warrants at an exercise price of $1, exercisable for 5 years from the issue date on the face of the Warrant. The maturity date of the loan was February 19, 2022. In May 2022, the Company and the noteholder agreed to cancel and void previous warrants and entered into a new agreement for 115,185 warrants with an exercise price of $2.50. The noteholder elected to convert the loan into 21,747 shares of common stock upon the Company’s initial public offering in February 2023.
On June 15, 2021, the company entered into a unsecured short term loan agreement with the Investor for an aggregate principal balance of $34,000, with a one-year maturity date, accruing interest at 5% and imputing an additional 1% interest. The full amount of the loan, including interest, was repaid in 2022.
On November 19, 2021, 2021, the company entered into an unsecured short term loan agreement with the Investor for an aggregate principal balance of $5,000, with a one-year maturity date, accruing interest at 5% and imputing an additional 1% interest. The full amount of the loan, including interest, was repaid in 2022.
On December 13, 2021, the company entered into an unsecured short term loan agreement with the Investor for an aggregate principal balance of $10,000, with a one-year maturity date, accruing interest at 5% and imputing an additional 1% interest. The full amount of the loan, including interest, was repaid in 2022.
On October 5, 2022, the Company entered into an exchange agreement with the Investor whereby all of his common stock, 734,493 shares, were exchanged into shares of Series A Convertible Preferred Stock. The Series A Preferred Stock is the economic equivalent of the common stock but has no voting rights and is subject to a blocker which prohibits the conversion into common stock if it would result in the Investor owning more than 4.99% of the Company’s outstanding common stock at such time. For a description of the rights and preferences of the Series A Preferred Stock, see “Description of Securities- Series A Convertible Preferred Stock”.
Related Person Transaction Policy
For purposes of our policy only, a related person transaction is a transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we and any related person are, were or will be participants in which the amount involved exceeds the lesser of $120,000 or 1% of the average of our total assets at year-end. Transactions involving compensation for services provided to us as an employee or director are not covered by this policy. A related person is any executive officer, director or beneficial owner of more than 5% of any class of our voting securities, including any of their immediate family members and any entity owned or controlled by such persons.
Under the policy, if a transaction has been identified as a related person transaction, including any transaction that was not a related person transaction when originally consummated or any transaction that was not initially identified as a related person transaction prior to consummation, our management must present information regarding the related person transaction to our audit committee, or, if audit committee approval would be inappropriate, to another independent body of our Board of Directors, for review, consideration and approval or ratification. The presentation must include a description of, among other things, the material facts, the interests, direct and indirect, of the related persons, the benefits to us of the transaction and whether the transaction is on terms that are comparable to the terms available to or from, as the case may be, an unrelated third party or to or from employees generally. Under the policy, we will collect information that we deem reasonably necessary from each director, executive officer and, to the extent feasible, significant stockholder to enable us to identify any existing or potential related-person transactions and to effectuate the terms of the policy. In addition, under our code of business conduct and ethics, our employees and directors will have an affirmative responsibility to disclose any transaction or relationship that reasonably could be expected to give rise to a conflict of interest. In considering related person transactions, our audit committee, or other independent body of our Board of Directors, will take into account the relevant available facts and circumstances including, but not limited to:
| ● | the risks, costs and benefits to us; | |
| ● | the impact on a director’s independence in the event that the related person is a director, immediate family member of a director or an entity with which a director is affiliated; | |
| ● | the availability of other sources for comparable services or products; and | |
| ● | the terms available to or from, as the case may be, unrelated third parties or to or from employees generally. |
The policy requires that, in determining whether to approve, ratify or reject a related person transaction, our audit committee, or other independent body of our Board of Directors, must consider, in light of known circumstances, whether the transaction is in, or is not inconsistent with, our best interests and those of our stockholders, as our audit committee, or other independent body of our Board of Directors, determines in the good faith exercise of its discretion.
| 57 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND
RELATED STOCKHOLDER MATTERS
The following table sets forth certain information regarding the beneficial ownership of our common stock as of January 25, 2024 by:
| ● | each of our named executive officers; | |
| ● | each of our directors; | |
| ● | all of our current directors and executive officers as a group; and | |
| ● | each stockholder known by us to own beneficially more than five percent of our common stock. |
Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities. Shares of common stock that may be acquired by an individual or group within 60 days of January 25, 2024, pursuant to the exercise of options or warrants and convertible debt are deemed to be outstanding for the purpose of computing the percentage ownership of such individual or group. Percentage of ownership is based on 6,094,644 shares of common stock outstanding on January 25, 2024, and 7,123,354 after giving effect to the sale of 1,028,710 shares in this offering.
Except as indicated in footnotes to this table, we believe that the stockholders named in this table have sole voting and investment power with respect to all shares of common stock shown to be beneficially owned by them, based on information provided to us by such stockholders. Unless otherwise indicated, the address of all listed stockholders is c/o Bullfrog AI Holdings, Inc., 325 Ellington Blvd., Unit 317, Gaithersburg, MD 20878.
| Name of Beneficial Owner | Common Stock Beneficially Owned | Percentage of Common Stock Before Offering | Percentage of Common Stock After Offering (1) | |||||||||
| Directors and Officers: | ||||||||||||
Vininder Singh (2) Chief Executive Officer and Director | 2,618,779 | 42.97 | % | 33.34 | % | |||||||
Dane Saglio (3) Chief Financial Officer | 108,642 | 1.76 | % | 1.38 | % | |||||||
| R. Don Elsey (4) | 21,110 | * | * | |||||||||
| William Enright (5) | 21,110 | * | * | |||||||||
| Jason Hanson (6) | 21,110 | * | * | |||||||||
| All officers and directors (5 persons) | 2,790,721 | 44.53 | % | 35.53 | % | |||||||
| Beneficial owners of more than 5% | ||||||||||||
| Tivoli Trust (7) | 904,391 | 13.020 | % | 10.42 | % | |||||||
*represents less than 1%.
| (1) | Assumes (i) no exercise by the underwriter of its option to purchase additional shares of common stock to cover over-allotments, if any; (ii) no exercise of the Warrants; and (iii) 1,028,710 shares of common stock and 478,429 prefunded warrants sold in this offering. (prefunded warrants which are reflected as issued shares) |
| (2) | Comprised of (i) 2,592,446 shares of common stock and (ii) 26,333 shares of common stock issuable upon exercise of common stock purchase stock options at an exercise price of $3.89 per shares. |
| (3) | Comprised of (i) 47,142 shares of common stock and (ii) 61,500 shares of common stock issuable upon exercise of common stock purchase stock options at a weighted average exercise price of $3.00 per share. |
| (4) | Includes 21,110 shares of common stock issuable upon exercise of stock options at an exercise price of $6.82 per share. |
| (5) | Includes 21,110 shares of common stock issuable upon exercise of stock options at an exercise price of $6.82 per share. |
| (6) | Includes 21,110 shares of common stock issuable upon exercise of stock options at an exercise price of $6.82 per share. |
| (7) | Comprised of (i) 73,449 shares of non-voting Series A Preferred Stock, (ii) 115,185 warrants exercisable at $2.50 per share of common stock, and (iii) 54,714 shares of common stock related to two convertible debt instruments that converted at a discount to the IPO price. Assumes the conversion of all Series A Preferred Stock into common stock in an amount equal to ten shares of common stock for each one share of Series A Preferred Stock. |
| 58 |
SHARES ELIGIBLE FOR FUTURE RESALE
Future sales of substantial amounts of our common stock in the public market or the perception that such sales might occur could adversely affect market prices prevailing from time to time. Furthermore, because only a limited number of shares will be available for sale shortly after this offering due to existing contractual and legal restrictions on resale as described below, there may be sales of substantial amounts of our common stock in the public market after the restrictions lapse. This may adversely affect the prevailing market price of our common stock and our ability to raise equity capital in the future.
After completion of this Offering, we will have 7,123,354 shares of common stock outstanding (or 7,349,425 shares if the underwriters’ option to purchase additional Company Shares is exercised in full).
All of the shares of common stock sold in this offering will be freely tradable without restrictions or further registration under the Securities Act, unless the shares are purchased by our “affiliates” as that term is defined in Rule 144 and except certain shares that will be subject to the lock-up period described below after completion of this offering. Any shares owned by our affiliates may not be resold except in compliance with Rule 144 volume limitations, manner of sale and notice requirements, pursuant to another applicable exemption from registration or pursuant to an effective registration statement.
Any of the shares held by our directors, officers and holders of at least 10% of the Company’s outstanding securities will be subject to a ninety (90) day lock-up restriction described under “Underwriting” on page 60. Accordingly, there will be a corresponding increase in the number of shares that become eligible for sale after the lock-up period expires. As a result of these agreements, subject to the provisions of Rule 144 or Rule 701, shares will be available for sale in the public market as follows:
| ● | beginning on the date of this prospectus, all of the shares sold in this offering will be immediately available for sale in the public market (except as described above); | |
| ● | beginning ninety (90) days after this offering is completed, at the expiration of the lock-up period for our officers, directors and holders of at least 10% of the Company’s outstanding securities, 3,695,112 additional shares will become eligible for sale in the public market, all of which shares will be held by affiliates and subject to the volume and other restrictions of Rule 144 and Rule 701 as described below. |
Company Warrants
The registration statement of which this prospectus is a part also registers for sale warrants to purchase up to 1,507,139 shares of common stock (or 1,733,210 shares of common stock if the underwriter exercises its over-allotment option in full). The Company Warrants will be exercisable immediately upon issuance and will expire on the fifth anniversary of the original issuance date and have an assumed initial exercise price equal to $4.16.
Pre-funded Warrants
The registration statement of which this prospectus is a part also registers for sale 478,429 Pre-Funded Warrants to purchase up to 478,429 shares of common stock for an assumed offering price of $3.781 per Pre-Funded Warrant (the combined price per Company Share and Company Warrant being sold to the public in this Offering, minus $0.001). The Pre-Funded Warrants will be exercisable immediately upon issuance, may be exercised at any time until all of the Pre-Funded Warrants are exercised in full, at an exercise price equal to $0.001. Please see “Description of Capital Stock - Pre-funded Warrants” for a description of these warrants.
Underwriter’s Warrants
In addition to cash compensation, we have agreed to issue to the underwriter warrants to purchase 90,428 shares of common stock equal to 6% of the aggregate number of Company Shares and/or Pre-funded Warrants issued in this Offering (excluding shares sold to cover over-allotments, if any). The Underwriter’s Warrants have an exercise price per share of $4.16 (110% of the combined public offering price of each Company Share and Company Warrant). The Underwriter’s Warrants will be exercisable from time to time, in whole or in part, during the five-year period commencing 180 days from the closing of this Offering. We have registered up to 90,428 shares of common stock issuable upon the exercise of the Underwriter’s Warrants in the registration statement of which this prospectus is a part (excluding shares sold to cover over-allotments, if any).
| 59 |
Rule 144
In general, under Rule 144 as currently in effect, once we have been subject to public company reporting requirements for at least 90 days, a person who is not deemed to have been one of our affiliates for purposes of the Securities Act at any time during the 90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at least six months, including the holding period of any prior owner other than our affiliates, is entitled to sell those shares without complying with the manner of sale, volume limitation or notice provisions of Rule 144, subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than our affiliates, then that person would be entitled to sell those shares without complying with any of the requirements of Rule 144.
In general, under Rule 144, as currently in effect, our affiliates or persons selling shares on behalf of our affiliates are entitled to sell upon expiration of the lock-up agreements described above, within any three-month period, a number of shares that does not exceed the greater of:
| ● | 1% of the number of shares of our common stock then outstanding, which will equal approximately shares immediately after this offering; or | |
| ● | the average weekly trading volume of our common stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to that sale. |
Sales under Rule 144 by our affiliates or persons selling shares on behalf of our affiliates are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about us.
Rule 701
Rule 701 generally allows a stockholder who purchased shares of our common stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of our company during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation or notice provisions of Rule 144. Rule 701 also permits affiliates of our company to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required by that rule to wait until 90 days after the date of this prospectus before selling those shares pursuant to Rule 701 and are subject to the lock-up agreements described above.
UNDERWRITING
WallachBeth Capital LLC is acting as the sole book-running manager and the representative of the underwriters of this Offering (the “Representative”). Subject to the terms and conditions of the underwriting agreement between us and the Representative, we have agreed to sell to the underwriters and the underwriters have agreed to purchase from us, at the combined public offering price per Company Share and accompanying Company Warrant, less the underwriting discounts set forth on the cover page of this prospectus, the number of Company Shares and accompanying Company Warrants listed next to its name in the following table:
| Underwriter |
Number of Company Shares (or Pre-Funded Warrants in lieu thereof) and Accompanying Company Warrants | |||
| WallachBeth Capital LLC | 978,318 | |||
| Kingswood Capital Partners, LLC | 528,821 | |||
| Total | 1,507,139 | |||
The underwriters are committed to purchase all the Company Shares and accompanying Company Warrants offered by us other than those covered by the option to purchase additional Company Shares and accompanying Company Warrants described below, if they purchase any such securities. The obligations of the underwriters may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriters’ obligations are subject to customary conditions, representations and warranties contained in the underwriting agreement, such as receipt by the underwriters of officers’ certificates and legal opinions.
| 60 |
We have agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriters may be required to make in respect thereof.
The underwriters have offered the Units, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel and other conditions specified in the underwriting agreement. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Over-allotment Option
We have granted the Representative of the underwriters an option to purchase from us, at the public offering price, less the underwriting discounts and commissions, up to an additional 226,071 Company Shares (and/or Pre-funded Warrants issued in lieu of Company Shares), and/or 226,071 Company Warrants, in any combination thereof, less the underwriting discounts and commissions, within 45 days from the date of this prospectus to cover over-allotments, if any.
Discount and Commissions; Expenses
We have agreed to pay the underwriter a cash fee equal to eight percent (8%) of the aggregate sales price of securities sold. The following table shows the public offering price, underwriting discount and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by the underwriters of their over-allotment option.
| Per Company Share and accompanying Company Warrant | Per Company Share and accompanying Company Warrant | Total Without Over- Allotment Option | Total With Over- Allotment Option | |||||||||||||
| Public offering price | $ | 3.782 | $ | 3.781 | $ | 5,700,000 | $ | 6,555,000 | ||||||||
| Underwriting discount (8%) | $ | 0.303 | $ | 0.302 | $ | 456,000 | $ | 524,400 | ||||||||
| Proceeds, before expenses, to us | $ | 3.479 | $ | 3.478 | $ | 5,244,000 | $ | 6,030,600 | ||||||||
The underwriters propose to offer the Company Shares and accompanying Company Warrants offered by us to the public at the combined public offering price per Company Share and accompanying Company Warrant set forth on the cover of this prospectus. In addition, the underwriters may offer some of the securities to other securities dealers at such price less a concession of $0.53 per Company Share and accompanying Company Warrant. If all of the Company Shares offered by us are not sold at the combined public offering price, the underwriters may change the combined offering price and other selling terms by means of a supplement to this prospectus.
We have also agreed to reimburse the underwriters for reasonable out-of-pocket expenses not to exceed $115,000 in the aggregate whether or not there is a closing of this offering. We estimate that total expenses payable by us in connection with this offering, other than the underwriting discount will be approximately $254,000. In addition, we have also agreed to pay to the underwriters a non-accountable expense allowance in the amount of 1% of the gross offering amount (including shares purchased upon exercise of the over-allotment option).
The underwriting agreement, however, provides that in the event the offering is terminated, any advance expense deposits paid to the underwriters will be returned to the extent that offering expenses are not actually incurred in accordance with FINRA Rule 5110(g)(4)(A).
Underwriter’s Warrants
As additional compensation to the underwriter, upon consummation of this offering, we will issue to the Representative or its designees warrants to purchase 90,428 shares of our common stock, equal to 6% of the number of Company Shares and/or Pre-Funded Warrants issued in this Offering (excluding any shares sold to cover over-allotments, if any), at an exercise price of $4.16 per share, equal to 110% of the public offering price (the “Underwriter’s Warrants”).
The Underwriter’s Warrants and the underlying shares of common stock will not be exercised, sold, transferred, assigned, pledged or hypothecated or be the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the Underwriter’s Warrants by any person for a period of 180 days from the effective date of the registration statement (the “Effective Date”) for this Offering in accordance with FINRA Rule 5110(e)(1). The Underwriter’s Warrants will be exercisable, in whole or in part, commencing 180 days from the closing of this Offering and will expire on the fifth anniversary thereof. We have registered the Underwriter’s Warrant and up to 90,428 shares of common stock issuable upon the exercise of the Underwriter’s Warrants in the registration statement of which this prospectus is a part.
Right of Participation
We have granted to the Representative a right of participation whereby, for a period of nine (9) months from the closing of this Offering, the Company will grant to the Representative the right to act as lead underwriter or book-running manager or placement agent for each and every future public and private equity and debt offerings of the Company, or any successor to or any subsidiary of the Company in any U.S. stock exchange during such nine (9) month period, according to the mechanism to be agreed upon by the parties in the placement agency agreement. The right of first participation shall only be in effect if the Offering results in gross proceeds of at least $4,500,000.
| 61 |
Discretionary Accounts
The underwriters do not intend to confirm sales of the securities offered hereby to any accounts over which they have discretionary authority.
Indemnification
We have agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriters may be required to make in respect thereof.
Determination of Offering Price
The public offering price set forth on the cover page of this prospectus has been determined based upon arm’s-length negotiations between the underwriter and us based upon, among other things, the trading of our shares of common stock prior to the Offering.
Lock-Up Agreements
We and each of our officers, directors, and 10% or greater stockholders have agreed, subject to certain exceptions, not to offer, issue, sell, contract to sell, encumber, grant any option for the sale of or otherwise dispose of any shares of our common stock or other securities convertible into or exercisable or exchangeable for shares of our common stock for a period of ninety (90) days after this offering is completed without the prior written consent of the Representative. The lock-up will not prevent the scheduled sale of any shares by the Company’s chief executive officer pursuant to his 10b-5 plan.
The Representative may in its sole discretion and at any time without notice release some or all of the shares subject to lock-up agreements prior to the expiration of the lock-up period. When determining whether or not to release shares from the lock-up agreements, the Representative will consider, among other factors, the security holder’s reasons for requesting the release, the number of shares for which the release is being requested and market conditions at the time.
| 62 |
Price Stabilization, Short Positions and Penalty Bids
In connection with this offering the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate covering transactions and penalty bids in accordance with Regulation M under the Exchange Act:
| ● | Stabilizing transactions permit bids to purchase securities so long as the stabilizing bids do not exceed a specified maximum. |
| ● | Over-allotment involves sales by the underwriters of securities in excess of the number of securities the underwriters are obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of securities over-allotted by the underwriters is not greater than the number of securities that they may purchase in the over-allotment option. In a naked short position, the number of securities involved is greater than the number of securities in the over-allotment option. The underwriters may close out any covered short position by either exercising its over-allotment option and/or purchasing securities in the open market. |
| ● | Syndicate covering transactions involve purchases of the securities in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of securities to close out the short position, the underwriters will consider, among other things, the price of securities available for purchase in the open market as compared to the price at which they may purchase securities through the over-allotment option. A naked short position occurs if the underwriters sell more securities than could be covered by the over-allotment option. This position can only be closed out by buying securities in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the securities in the open market after pricing, that could adversely affect investors who purchased securities in this Offering. |
| ● | Penalty bids permit the underwriters to reclaim a selling concession from a syndicate member when securities originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our securities or preventing or retarding a decline in the market price of the securities. As a result, the price of our shares of common stock and warrants may be higher than the price that might otherwise exist in the open market. These transactions may be discontinued at any time.
Neither we nor the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our shares of common stock and warrants. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that any transaction, if commenced, will not be discontinued without notice.
Electronic Offer, Sale and Distribution of Shares
A prospectus in electronic format may be made available on a website maintained by the Representative and may also be made available on a website maintained by other underwriters. The underwriters may agree to allocate a number of shares to underwriters for sale to their online brokerage account holders. Internet distributions will be allocated by the Representative to underwriters that may make Internet distributions on the same basis as other allocations. In connection with the offering, the underwriters or syndicate members may distribute prospectuses electronically. No forms of electronic prospectus other than prospectuses that are printable as Adobe® PDF will be used in connection with this offering.
The underwriters have informed us that they do not expect to confirm sales of shares offered by this prospectus to accounts over which they exercise discretionary authority.
Other than the prospectus in electronic format, the information on any underwriter’s website and any information contained in any other website maintained by an underwriter is not part of the prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or any underwriter in its capacity as underwriter and should not be relied upon by investors.
| 63 |
Trading; Nasdaq Capital Market Listing
Our common stock and tradeable warrants are listed on The Nasdaq Capital Market under the symbols “BFRG” and “BFRGW,” respectively. We have not applied, and do not intend to apply, to list the Pre-Funded Warrants or the Company Warrants on Nasdaq.
Other Relationships
From time to time, the underwriters and/or their affiliates have provided, and may in the future provide, various investment banking and other financial services for us for which services it has received and, may in the future receive, customary fees. Except for the services provided in connection with this Offering and other than as described below, the underwriters have not provided any investment banking or other financial services during the 180-day period preceding the date of this prospectus.
Offers Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
EXPERTS
The financial statements of Bullfrog AI Holdings, Inc. for the fiscal years ended December 31, 2022 and 2021, as set forth in this prospectus and elsewhere in the registration statement have been so included in reliance on the reports herein of M&K CPAs, an independent registered public accounting firm, given on their authority as experts in accounting and auditing.
LEGAL MATTERS
Sichenzia Ross Ference Carmel LLP, New York, New York, will pass upon the validity of the shares of our common stock to be sold in this offering. Sheppard, Mullin, Richter & Hampton LLP, New York, New York, will pass upon certain legal matters for the underwriters.
| 64 |
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the securities we are offering to sell. This prospectus, which constitutes part of the registration statement, does not include all of the information contained in the registration statement and the exhibits, schedules and amendments to the registration statement. For further information with respect to us and our securities, we refer you to the registration statement and to the exhibits and schedules to the registration statement. Statements contained in this prospectus about the contents of any contract, agreement or other document are not necessarily complete, and, in each instance, we refer you to the copy of the contract, agreement or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
The SEC maintains a website, which is located at www.sec.gov, that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. You may access the registration statement of which this prospectus is a part at the SEC’s website.
Upon completion of this offering, we will be subject to the information reporting requirements of the Securities Exchange Act of 1934, and we will file reports, proxy statements and other information with the SEC. All documents filed with the SEC are available for inspection and copying at the public reference room and website of the SEC referred to above. We maintain a website at www.bullfrogai.com. You may access our reports, proxy statements and other information free of charge at this website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information on such website is not incorporated by reference and is not a part of this prospectus.
| 65 |
BULLFROG AI HOLDINGS, INC.
INDEX TO FINANCIAL STATEMENTS
| Audited Financial Statements for the Years Ended December 31, 2022 and 2021 | |
| Report of Independent Registered Public Accounting Firm | F-2 |
| Consolidated Balance Sheets | F-3 |
| Consolidated Statements of Operations | F-4 |
| Consolidated Statements of Shareholders’ Deficit | F-5 |
| Consolidated Statements of Cash Flows | F-6 |
| Notes to Consolidated Financial Statements | F-7 |
| Unaudited Condensed Consolidated Financial Statements for the Three and Nine Months Ended September 30, 2023 and 2022 | |
| Condensed Consolidated Balance Sheets | F-21 |
| Condensed Consolidated Statements of Operations | F-22 |
| Condensed Consolidated Statements of Changes in Stockholders’ Deficit | F-23 |
| Condensed Consolidated Statements of Cash Flows | F-24 |
| Notes to Condensed Consolidated Financial Statements | F-25 |
| F-1 |
BULLFROG AI HOLDINGS, INC.
AUDITED FINANCIAL STATEMENTS
2022 and 2021
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Bullfrog AI Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Bullfrog AI Holdings, Inc. (the Company) as of December 31, 2022 and 2021, and the related consolidated statements of operations, changes in stockholders’ deficit, and cash flows for the years ended December 31, 2022 and 2021, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021 and the results of its operations and its cash flows for flows for the two-year period ended December 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of a critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which they relate.
As discussed in Note 2, the Company had a going concern disclosure in the previous year due to continued net losses from operations and negative cash flows in operations. Auditing management’s evaluation of a going concern can be a significant judgment given the fact that the Company uses management estimates on future revenues and expenses, which are difficult to substantiate.
We evaluated the appropriateness of the removal of the going concern, we examined and evaluated the financial information along with management’s plans to mitigate the going concern and management’s disclosure on going concern.
| /s/ M&K CPAS, PLLC | |
| We have served as the Company’s auditor since 2021. | |
| Houston, Texas | |
| April 25, 2023 |
| F-2 |
BULLFROG AI HOLDINGS, INC.
CONSOLIDATED BALANCE SHEETS
| December 31 | December 31 | |||||||
| 2022 | 2021 | |||||||
| (Audited) | (Audited) | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | 57,670 | $ | 10,014 | ||||
| Prepaid expense | 15,000 | - | ||||||
| Total Current Assets | $ | 72,670 | $ | 10,014 | ||||
| NON-CURRENT ASSETS: | ||||||||
| Property and Equipment, net | 7,699 | - | ||||||
| Total Non-Current Assets | $ | 7,699 | - | |||||
| TOTAL ASSETS | $ | 80,369 | $ | 10,014 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable | $ | 543,993 | $ | 68,594 | ||||
| Accrued expenses | 416,072 | 68,557 | ||||||
| Accrued expenses-related party | 566,916 | 285,666 | ||||||
| Deferred revenue | 32,000 | 10,000 | ||||||
| Notes payable-related party | - | 49,000 | ||||||
| Convertible notes, net of $0 and $12,962 debt discount, respectively | 1,323,890 | 284,038 | ||||||
| Convertible notes-related party, net of $0 and $1,584 debt discount, respectively | 254,850 | 253,266 | ||||||
| Total Current Liabilities | $ | 3,137,721 | $ | 1,019,121 | ||||
| TOTAL LIABILITIES | $ | 3,137,721 | $ | 1,019,121 | ||||
| STOCKHOLDERS’ DEFICIT: | ||||||||
| Series A Preferred stock, $0.00001 par value, 5,500,000 shares authorized; 73,449 and 0 shares are issued and outstanding, respectively, | 1 | - | ||||||
| Common stock, $0.00001 par value, 100,000,000 shares authorized; 4,021,935 and 4,622,789 shares are issued and outstanding as of December 31, 2022 and 2021, respectively | 40 | 46 | ||||||
| Additional paid-in capital | 1,341,662 | 587,415 | ||||||
| Accumulated deficit | (4,399,055 | ) | (1,596,568 | ) | ||||
| Total BullFrog stockholders’ deficit | $ | (3,057,352 | ) | $ | (1,009,107 | ) | ||
| TOTAL STOCKHOLDERS’ DEFICIT | (3,057,352 | ) | (1,009,107 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | 80,369 | $ | 10,014 | ||||
The accompanying notes are an integral part of these financial statements
| F-3 |
BULLFROG AI HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| For Years Ended December 31 | ||||||||
| 2022 | 2021 | |||||||
| NET REVENUES: | ||||||||
| Revenues, net | $ | 10,000 | $ | - | ||||
| TOTAL NET REVENUES | $ | 10,000 | - | |||||
| COST OF GOODS SOLD: | ||||||||
| Cost of goods sold | 800 | - | ||||||
| TOTAL COST OF GOODS SOLD | 800 | - | ||||||
| GROSS PROFIT | 9,200 | - | ||||||
| OPERATING EXPENSES: | ||||||||
| Research and development expenses | 609,270 | 25,000 | ||||||
| General and administrative expenses | 1,307,882 | 327,329 | ||||||
| Payroll and salary | 98,250 | - | ||||||
| Payroll and salary-related party | 449,599 | 203,033 | ||||||
| TOTAL OPERATING EXPENSES | 2,465,001 | 555,362 | ||||||
| (LOSS) FROM OPERATIONS | (2,455,801 | ) | (555,362 | ) | ||||
| OTHER INCOME (EXPENSE): | ||||||||
| Interest expense | (347,145 | ) | (40,395 | ) | ||||
| Other Income | 459 | 9,917 | ||||||
| TOTAL OTHER (EXPENSE) | (346,686 | ) | (30,478 | ) | ||||
| NET (LOSS) | (2,802,487 | ) | (585,840 | ) | ||||
| NET (LOSS) PER COMMON SHARE: | ||||||||
| Basic and diluted | $ | (0.70 | ) | $ | (014 | ) | ||
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING: | ||||||||
| Basic and diluted | 4,009,852 | 4,116,336 | ||||||
The accompanying notes are an integral part of these financial statements
| F-4 |
BULLFROG AI HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ (DEFICIENCY) EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2022 AND 2021
| Series A Preferred stock | Common Stock | Additional Paid in | Subscription | Accumulated | ||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Receivables | Deficit | Total | |||||||||||||||||||||||||
| Balances, December 31, 2020 | - | $ | - | 3,603,422 | $ | 36 | $ | 470,274 | $ | (100 | ) | $ | (1,010,728 | ) | $ | (540,518 | ) | |||||||||||||||
| Cash from subscription receivables | - | - | - | - | - | 100 | - | 100 | ||||||||||||||||||||||||
| Warrants issued with convertible notes | - | - | - | - | 13,661 | - | - | 13,661 | ||||||||||||||||||||||||
| Imputed Interest | - | - | - | - | 4,539 | - | - | 4,539 | ||||||||||||||||||||||||
| Equity compensation | - | - | - | - | 9,385 | - | - | 9,385 | ||||||||||||||||||||||||
| Equity compensation | - | - | 1,019,367 | 10 | 89,556 | - | - | 89,566 | ||||||||||||||||||||||||
| - | ||||||||||||||||||||||||||||||||
| Net (Loss) | - | - | - | - | - | - | (585,840 | ) | (585,840 | ) | ||||||||||||||||||||||
| Balances, December 31, 2021 | - | $ | - | 4,622,789 | $ | 46 | $ | 587,415 | - | $ | (1,596,568 | ) | $ | (1,009,107 | ) | |||||||||||||||||
| Imputed Interest | - | - | - | - | 9,221 | - | - | 9,221 | ||||||||||||||||||||||||
| Equity compensation | - | - | - | - | 340,152 | - | - | 340,152 | ||||||||||||||||||||||||
| Conversion of convertible notes | - | - | 205,984 | 2 | 226,136 | - | - | 226,138 | ||||||||||||||||||||||||
| Reclassification of warrant | - | - | - | - | (11,097 | ) | - | - | (11,097 | ) | ||||||||||||||||||||||
| Shares cancellation | - | - | (112,225 | ) | (1 | ) | 1 | - | - | - | ||||||||||||||||||||||
| Shares issuance for license | - | - | 39,879 | - | 189,828 | - | - | 189,828 | ||||||||||||||||||||||||
| Common stocks converted to Series A Preferred stock | 73,449 | 1 | (734,492 | ) | (7 | ) | 6 | - | - | - | ||||||||||||||||||||||
| Net (Loss) | - | - | - | - | - | - | (2,802,487 | ) | (2,802,487 | ) | ||||||||||||||||||||||
| Balances, December 31, 2022 | 73,449 | 1 | 4,021,935 | $ | 40 | 1,341,662 | $ | - | $ | (4,399,055 | ) | $ | (3,057,352 | ) | ||||||||||||||||||
The accompanying notes are an integral part of these financial statements
| F-5 |
BULLFROG AI HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOW
| For The Years Ended December 31 | ||||||||
| 2022 | 2021 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net (loss) | $ | (2,802,487 | ) | $ | (585,840 | ) | ||
| Adjustment to reconcile change in net (loss) to net cash and cash equivalents used in operating activities: | ||||||||
| Gain on debt forgiveness | - | (9,917 | ) | |||||
| Depreciation expense | 1,045 | - | ||||||
| Shares issuance for license | 189,828 | - | ||||||
| Stock-based compensation | 340,152 | 98,951 | ||||||
| Amortization of debt discount | 214,429 | 12,665 | ||||||
| Imputed Interest | 9,221 | 4,539 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid Expense | (15,000 | ) | - | |||||
| Accounts payable | 475,399 | (25,853 | ) | |||||
| Accrued expenses | 373,273 | 27,384 | ||||||
| Accrued expenses-related party | 281,250 | 85,666 | ||||||
| Deferred revenue | 22,000 | 10,000 | ||||||
| NET CASH USED IN OPERATING ACTIVITIES | (910,890 | ) | (382,405 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchase of Property and Equipment | (8,744 | ) | - | |||||
| NET CASH FROM INVESTING ACTIVITIES | (8,744 | ) | - | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from convertible notes payables | 1,016,290 | - | ||||||
| Proceeds from convertible notes payables-related party | - | 298,900 | ||||||
| Repayment of note payable and interest-related party | (49,000 | ) | - | |||||
| Proceeds from notes payables - related party | - | 88,400 | ||||||
| Proceeds from subscription payable | - | 100 | ||||||
| NET CASH FROM FINANCING ACTIVITIES | 967,290 | 387,400 | ||||||
| Net increase/(decrease) in cash and cash equivalents | 47,656 | 4,995 | ||||||
| Cash, beginning of year | 10,014 | 5,019 | ||||||
| Cash, end of period | $ | 57,670 | $ | 10,014 | ||||
| SUPPLEMENTAL CASH FLOW INFORMATION: | ||||||||
| Cash paid for interest | $ | 5,757 | $ | - | ||||
| Cash paid for taxes | $ | - | $ | - | ||||
| SUPPLEMENTAL DISCLOSURE of NON-CASH ACTIVITY: | ||||||||
| Reclassification of warrant | $ | 11,097 | $ | - | ||||
| Conversion of Convertible Note payable | $ | 226,138 | $ | - | ||||
| Cancellation of common stocks | $ | 8 | $ | - | ||||
| Shares issued for license | $ | 189,828 | $ | - | ||||
| Shares issued for services | $ | 340,152 | $ | 20 | ||||
| Warrants issued with convertible notes | $ | - | $ | 13,661 | ||||
The accompanying notes are an integral part of these financial statements
| F-6 |
BULLFROG AI HOLDINGS, INC.
NOTES TO FINANCIAL STATEMENTS
December 31, 2022 and 2021
NOTE 1 - ORGANIZATION AND NATURE OF BUSINESS
Organization and Nature of Business
Bullfrog AI Holdings, Inc. was incorporated in the State of Nevada on February 6, 2020. Bullfrog AI Holdings, Inc. is the parent company of Bullfrog AI, Inc. and Bullfrog AI Management, LLC. which were incorporated in Delaware and Maryland, in 2017 and 2021, respectively. All of our operations are currently conducted through BullFrog AI Holdings, Inc., which began operations on February 6, 2020. We are a company focused specifically on advanced AI/ML-driven analysis of complex data sets in medicine and healthcare. Our objective is to utilize our platform for precision medicine approach to drug asset enablement through external partnerships and selective internal development.
Most new therapeutics will fail at some point in preclinical or clinical development. This is the primary driver of the high cost of developing new therapeutics. A major part of the difficulty in developing new therapeutics is efficient integration of complex and highly dimensional data generated at each stage of development to de-risk subsequent stages of the development process. Artificial Intelligence and Machine Learning (AI/ML) has emerged as a digital solution to help address this problem.
We use artificial intelligence and machine learning to advance medicines for both internal and external projects. Most current AI/ML platforms still fall short in their ability to synthesize disparate, high-dimensional data for actionable insight. Our platform technology, named, bfLEAP™ is an analytical AI/ML platform developed at The Johns Hopkins University Applied Physics Laboratory (JHU-APL) which is able to surmount the challenges of scalability and flexibility currently hindering researchers and clinicians by providing a more precise, multi-dimensional understanding of their data. We are deploying bfLEAP™ for use at several critical stages of development for internal programs and through strategic partnerships and collaborations with the intention of streamlining data analytics in therapeutics development, decreasing the overall development costs by decreasing failure rates for new therapeutics, and impacting the lives of countless patients that may otherwise not receive the therapies they need.
The bfLEAP™ platform utilizes both supervised and unsupervised machine learning - as such, it is able to reveal real/meaningful connections in the data without the need for an a priori hypothesis. Algorithms used in the bfLEAP™ platform are designed to handle highly imbalanced data sets to successfully identify combinations of factors that are associated with outcomes of interest.
Our primary goal is to improve the odds of success at any stage of pre-clinical and clinical therapeutics development, for in house programs, and our strategic partners and collaborators. Our primary business model is enabling the success of ongoing clinical trials or rescue of late stage failed drugs (i.e., Phase 2 or Phase 3 clinical trial failures) for development and divestiture; although, we will also consider collaborations for earlier stage drugs. We hope to accomplish this through strategic acquisitions of current clinical stage and failed drugs for in-house development, or through strategic partnerships with biopharmaceutical industry companies. We are able to pursue our drug asset enhancement business by leveraging a powerful and proven AI/ML platform (trade name: bfLEAP™) initially developed at JHU-APL. We believe the bfLEAP™ analytics platform is a potentially disruptive tool for analysis of pre-clinical and/or clinical data sets, such as the robust pre-clinical and clinical trial data sets being generated in translational R&D and clinical trial settings.
NOTE 2-SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Summary of Significant Accounting Policies
Use of Estimates in the Preparation of Financial Statements
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires us to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Estimates include, but are not limited to, revenue recognition, allowances for doubtful accounts, recoverability of deferred tax assets and certain other of our accrued liabilities. Actual results could differ from those estimates.
| F-7 |
Financial Instruments
The carrying value of short-term instruments, including cash and cash equivalents, accounts payable and accrued expenses approximate fair value due to the relatively short period to maturity for these instruments.
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value maximize the use of observable inputs and minimize the use of unobservable inputs. The Company utilizes a three-level valuation hierarchy for disclosures of fair value measurements, defined as follows:
Level 1 - inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level 2 - inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments.
Level 3 - inputs to the valuation methodology are unobservable and significant to the fair value.
The Company does not have any assets or liabilities that are required to be measured and recorded at fair value on a recurring basis.
Revenue Recognition
For annual reporting periods after December 15, 2017, the Financial Accounting Standards Board (“FASB”) made effective ASU 2014-09 “Revenue from Contracts with Customers,” to supersede previous revenue recognition guidance under current U.S. GAAP. Revenue is now recognized in accordance with FASB ASC Topic 606, Revenue Recognition. The objective of the guidance is to establish the principles that an entity shall apply to report useful information to users of financial statements about the nature, amount, timing, and uncertainty of revenue and cash flows arising from a contract with a customer. The core principle is to recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the Company expects to be entitled in exchange for those goods or services. Two options were made available for implementation of the standard: the full retrospective approach or modified retrospective approach. The guidance became effective for annual reporting periods beginning after December 15, 2017, including interim periods within that reporting period, with early adoption permitted. We have adopted FASB ASC Topic 606 for our reporting period as of the year-ended December 31, 2019. As of December 31, 2021, we have had no revenue. In Q4 2022 the Company recognized its first service revenues in the amount of $10,000 related to the achievement of a contract milestone under a contract with a Pharmaceutical company. In compliance with the agreement, we have met the following milestones - receipt of data for analysis; data conversion and staging for ingestion. For the years-ended December 31, 2022 and 2021, our balance sheet reflects customer down payment received in early 2022 and late 2021 as unearned revenue in the amount of $32,000 and $10,000, respectively. This unearned revenue represents payments received from a leading rare disease non-profit organization under a contract with a single deliverable. As is more fully discussed below, we are of the opinion that none of our contracts for products contain significant financing components that require revenue adjustment under FASB ASC Topic 606.
Revenue is recognized based on the following five step model:
| - | Identification of the contract with a customer This step outlines the criteria that must be met when establishing a contract with a customer to supply goods or services | |
| - | Identification of the performance obligations in the contract This step describes how distinct performance obligations in the contract must be handled | |
| - | Determination of the transaction price This step outlines what must be considered when establishing the transaction price, which is the amount the business expects to receive for transferring the goods and services to the customer | |
| - | Allocation of the transaction price to the performance obligations in the contract This step outlines guidelines for allocating the transaction price across the contract’s separate performance obligations, and is what the customer agrees to pay for the goods and services | |
| - | Recognition of revenue when, or as, the Company satisfies a performance obligation Revenue can be recognized as the business meets each performance obligation. This step specifies how that should happen |
| F-8 |
Contract Services
The Company anticipates that the majority of revenues to be recognized in the near future will result from our fee for service partnership offering, designed for biopharmaceutical companies, as well as other organizations, of all sizes that have challenges analyzing data throughout the drug development process. The Company provides the customer with an analysis of large complex data sets using the Company’s proprietary Artificial Intelligence / Machine Learning platform called bfLEAP™. This platform is designed to predict targets of interest, patterns, relationships, and anomalies. The Company believes that there will be additional on-going work requested from partners therefore the service model utilizes a master services agreement with work or task orders issued for discrete analysis performed at the discovery, preclinical, or clinical stages of drug development. The Company receives a cash fee and in some instances the potential for rights to new intellectual property generated from the analysis.
Collaborative Arrangements
The Company also intends to enter collaborative arrangements with pharmaceutical companies who have drugs that have failed late Phase 2 or Phase 3 trials. These arrangements could take several forms including true partnerships where BullFrog contributes data analysis using the bfLEAP™ platform with the partner contributing the drug candidate and other resources needed to continue development towards commercialization with BullFrog receiving an equity or royalty right in the commercialized product. In other arrangements the Company may earn cash payments based on achieving certain milestones as determined under each specific arrangement.
Acquisition of Rights to Certain Drugs
In certain circumstances, we may also acquire rights to drugs that are in early-stage clinical trials, use our technology to sponsor and support a successful later stage precision medicine trial, and divest the asset. The same process may apply to the discovery of new drugs. In these instances, divestiture may be in the form of an outright sale of all rights or possibly a license to develop and commercialize enhanced development candidates. License agreements could include developmental and commercial milestones in addition to royalties.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant estimates include the fair value of the Company’s stock, stock-based compensation, fair values relating to derivative liabilities, debt discounts and the valuation allowance related to deferred tax assets. Actual results may differ from these estimates.
Cash
The Company considers cash to consist of cash on hand and temporary investments having an original maturity of 90 days or less that are readily convertible into cash. As of December 31, 2022 and 2021, cash balances were $57,670 and $10,014, respectively.
Concentrations of Credit Risk
The Company’s financial instruments that are exposed to a concentration of credit risk are cash and accounts receivable. Occasionally, the Company’s cash in interest-bearing accounts may exceed FDIC insurance limits. The financial stability of these institutions is periodically reviewed by senior management.
| F-9 |
Accounts Receivable
Trade receivables are carried at their estimated collectible amounts. Trade credit is generally extended on a short-term basis. Thus, trade receivables do not bear interest. Trade accounts receivable are periodically evaluated for collectability based on past credit history with customers and their current financial condition.
Allowance for Doubtful Accounts
Any charges to the allowance for doubtful accounts on accounts receivable are charged to operations in amounts sufficient to maintain the allowance for uncollectible accounts at a level management believes is adequate to cover any probable losses. Management determines the adequacy of the allowance based on historical write-off percentages and the current status of accounts receivable. Accounts receivables are charged off against the allowance when collectability is determined to be permanently impaired. As of December 31, 2022 and 2021, allowance for doubtful accounts was $0.
Inventories
The Company does not have inventory and does not plan to have inventory in the near future.
Cost of Sales
Cost of sales is comprised of royalties and the cost of outsourced services provided to the Company related to customer service contracts. We recognized $800 as cost of goods sold which represents the 8% royalty on the $10,000 in service revenue in 2022.
Property and Equipment
Property and equipment are stated at cost. When retired or otherwise disposed, the related carrying value and accumulated depreciation are removed from the respective accounts and the net difference less any amount realized from disposition, is reflected in earnings. For financial statement purposes, property and equipment are recorded at cost and depreciated using the straight-line method over their estimated useful lives.
Advertising
The Company follows the policy of charging the costs of advertising to expense as incurred.
Income Taxes
Deferred income tax assets and liabilities are determined based on the estimated future tax effects of net operating loss and credit carry forwards and temporary differences between the tax basis of assets and liabilities and their respective financial reporting amounts measured at the current enacted tax rates. The Company records an estimated valuation allowance on its deferred income tax assets if it is not more likely than not that these deferred income tax assets will be realized.
The Company recognizes a tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by taxing authorities, based on the technical merits of the position. The tax benefits recognized in the condensed consolidated financial statements from such a position are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement. As of December 31, 2022 and 2021, the Company has not recorded any unrecognized tax benefits.
Stock-Based Compensation
Employee and non-employee share-based compensation is measured at the grant date, based on the fair value of the award, and is recognized as an expense over the requisite service period.
Net Loss per Share
We compute net loss per share in accordance with ASC 260, Earning per Share. We report both basic and diluted loss per share. Loss earnings per share is calculated based on the weighted average number of shares of common stock outstanding and excludes the dilutive effect of warrants, stock options or any other type of convertible securities. Considering that the Common shares of the Company were not publicly traded as of December 31, 2022, the contingently convertible notes and related dilutive shares are not included in the dilutive shares calculation upon the Initial Public Offering (IPO). Diluted loss per share is calculated based on the weighted average number of shares of common stock outstanding and the dilutive effect of stock options, warrants and other types of convertible securities are included in the calculation. Dilutive securities are excluded from the diluted earnings per share calculation because their effect is anti-dilutive. As of December 31, 2021 and December 31, 2022, 927,373 and 753,174 warrants (post reverse stock split) were not included in the calculation of net loss per share, respectively. In addition, 486,571 and 56,242 options for common shares (post reverse stock split) were not included in the calculation of net loss per share, respectively.
| F-10 |
Recent Accounting Pronouncements
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842). This ASU requires lessees to recognize a lease liability, on a discounted basis, and a right-of-use asset for substantially all leases, as well as additional disclosures regarding leasing arrangements. In July 2018, the FASB issued ASU 2018-11, Leases (Topic 842), which provides an optional transition method of applying the new lease standard. Topic 842 can be applied using either a modified retrospective approach at the beginning of the earliest period presented, or as permitted by ASU 2018-11, at the beginning of the period in which it is adopted.
We adopted this standard using a modified retrospective approach since inception of the company. The modified retrospective approach includes a number of optional practical expedients relating to the identification and classification of leases that commenced as of the inception of the company; initial direct costs for leases that commenced as of inception of the company; and the ability to use hindsight in evaluating lessee options to extend or terminate a lease or to purchase the underlying asset.
The Company elected the package of practical expedients permitted under ASC 842 allowing it to account for its prior operating lease that commenced before the adoption date as an operating lease under the new guidance without reassessing (i) whether the contract contains a lease; (ii) the classification of the lease; or (iii) the accounting for indirect costs as defined in ASC 842.
All staff are working remotely; therefore, the Company does not currently have a lease or rent office space.
Consistent with ASC 842-20-50-4, the Company’s financial statements for the years ended December 31, 2022 and 2021, do not have a monthly rent obligation. The Company had no cash flows arising from a lease, no finance lease cost, short term lease cost, or variable lease costs. The Company does not produce any sublease income or any net gain or loss recognized from sale and leaseback transactions. As a result, the Company did not need to segregate amounts between finance and operating leases for cash paid for amounts included in the measurement of lease liabilities, segregated between operating and financing cash flows; supplemental non-cash information on lease liabilities arising from obtaining right-of-use assets; weighted-average calculations for the remaining lease term; or the weighted-average discount rate.
The adoption of this guidance resulted in no significant impact to the Company’s results of operations or cash flows.
In December 2019, the FASB issued ASU No. 2019-12 - Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes (“ASU 2019-12”). ASU 2019-12 is part of the FASB’s overall simplification initiative and seeks to simplify the accounting for income taxes by updating certain guidance and removing certain exceptions. The updated guidance is effective for fiscal years beginning after December 15, 2020 and interim periods within those fiscal years. Early adoption is permitted. The adoption of this update did not have a material effect on the Company’s financial statements.
In August 2020, the FASB issued ASU 2020-06, Debt - Debt with Conversion and Other Options (Subtopic 470- 20) and Derivatives and Hedging - Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”), which simplifies the accounting for certain financial instruments with characteristics of liabilities and equity. This ASU (1) simplifies the accounting for convertible debt instruments and convertible preferred stock by removing the existing guidance in ASC 470-20, Debt: Debt with Conversion and Other Options, that requires entities to account for beneficial conversion features and cash conversion features in equity, separately from the host convertible debt or preferred stock; (2) revises the scope exception from derivative accounting in ASC 815-40 for freestanding financial instruments and embedded features that are both indexed to the issuer’s own stock and classified in stockholders’ equity, by removing certain criteria required for equity classification; and (3) revises the guidance in ASC 260, Earnings Per Share, to require entities to calculate diluted earnings per share (EPS) for convertible instruments by using the if-converted method. In addition, entities must presume share settlement for purposes of calculating diluted EPS when an instrument may be settled in cash or shares. For SEC filers, excluding smaller reporting companies, ASU 2020-06 is effective for fiscal years beginning after December 15, 2021 including interim periods within those fiscal years. Early adoption is permitted, but no earlier than fiscal years beginning after December 15, 2020. For all other entities, ASU 2020-06 is effective for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. Entities should adopt the guidance as of the beginning of the fiscal year of adoption and cannot adopt the guidance in an interim reporting period. The Company elected early adoption, effective January 1, 2021. Considering that the Common shares of the Company were not publicly traded as of December 31, 2022, the convertible options are not considered to be readily convertible to cash. In addition, the beneficial conversion feature was eliminated under ASU 2020-06. Therefore, no derivative liabilities will be triggered from these convertible notes.
In October 2020, the FASB issued ASU 2020-10, Codification Improvements, which updates various codification topics by clarifying or improving disclosure requirements to align with the SEC’s regulations. The Company adopted ASU 2020-10 as of the reporting period beginning January 1, 2021. The adoption of this update did not have a material effect on the Company’s financial statements.
| F-11 |
The Company does not believe that any other recently issued effective pronouncements, or pronouncements issued but not yet effective, if adopted, would have a material effect on the accompanying financial statements.
COVID-19
In March 2020, the World Health Organization declared the global emergence of the COVID-19 pandemic. The impact of COVID-19 on the Company’s business is currently unknown. The Company will continue to monitor guidance and orders issued by federal, state, and local authorities with respect to COVID-19. As a result, the Company may take actions that alter its business operations as may be required by such guidance and orders or take other steps that the Company determines are in the best interest of its employees, customers, partners, suppliers and stockholders.
Any such alterations or modifications could cause substantial interruption to the Company’s business and could have a material adverse effect on the Company’s business, operating results, financial condition, and the trading price of the Company’s common stock, and could include temporary closures of one or more of the Company’s facilities; temporary or long-term labor shortages; temporary or long-term adverse impacts on the Company’s supply chain and distribution channels; and the potential of increased network vulnerability and risk of data loss resulting from increased use of remote access and removal of data from the Company’s facilities. In addition, COVID-19 could negatively impact capital expenditures and overall economic activity in the impacted regions or depending on the severity, globally, which could impact the demand for the Company’s products and services.
It is unknown whether and how the Company may be impacted if the COVID-19 pandemic persists for an extended period of time or if there are increases in its breadth or in its severity, including as a result of the waiver of regulatory requirements or the implementation of emergency regulations to which the Company is subject. The COVID-19 pandemic poses a risk that the Company or its employees, contractors, suppliers, and other partners may be prevented from conducting business activities for an indefinite period.
The Company may incur expenses or delays relating to such events outside of its control, which could have a material adverse impact on its business, operating results, financial condition and the trading price of its common stock.
Going Concern
The Company has had negative cash flows from operations and operated at a net loss since inception. In the prior year our auditors included a paragraph in their opinion regarding the substantial doubt that existed of our ability to continue as a going concern. As noted in note 14 we completed our initial public offering subsequent to year end. We believe that the funds raised and notes that were converted from debt to equity now provides enough liquidity to alleviate the substantial doubt. There can be no assurance that we will not need additional funding in the future.
NOTE 3 -PROPERTY AND EQUIPMENT
Property and Equipment
Property and equipment consisted of the following:
During the year ended December 31, 2022, the Company acquired $8,744 of equipment and has accumulated depreciation of $1,045, for a net of $7,699.
Depreciation expense totaled $1,045, and $0 in the years ended December 31, 2022 and 2021, respectively.
NOTE 4 -ACCOUNTS PAYABLE AND ACCRUED EXPENSES
Accounts Payable and Accrued Expenses
As of December 31, 2022 and 2021, the Company had accounts payable and accrued expenses totaling $1,526,981 and $422,817, respectively.
NOTE 5 -NOTES PAYABLE
Notes Payable
On May 5, 2020 the Company received an SBA PPP loan in the amount of $9,917, at 1% interest. The loan was forgiven on March 15, 2021.
NOTE 6 -NOTES PAYABLE RELATED PARTY
Notes Payable Related Party
On June 15, 2021, the company entered into an unsecured short term loan agreement with a related party for an aggregate principal balance of $34,000, with a one-year maturity date, accruing interest at 5% and imputing an additional 1% interest. The full amount of the loan and interest was repaid in 2022.
On November 19, 2021, 2021, the company entered into an unsecured short term loan agreement with a related party for an aggregate principal balance of $5,000, with a one-year maturity date, accruing interest at 5% and imputing an additional 1% interest. The full amount of the loan and interest was repaid in 2022.
On December 13, 2021, the company entered into an unsecured short term loan agreement with a related party for an aggregate principal balance of $10,000, with a one-year maturity date, accruing interest at 5% and imputing an additional 1% interest. The full amount of the loan and interest was repaid in 2022.
| F-12 |
NOTE 7 - CONVERTIBLE NOTES PAYABLE
Convertible Notes Payable
On March 27, 2020, the company entered into a convertible loan agreement with the Maryland Technology Development Corporation with a principal balance of $200,000 at 6% interest. The maturity date of the loan was September 27, 2021. During the year ended December 31, 2022, the full amount of the loan and interest totaling $226,138 was converted into 205,984 shares of common stock (post reverse stock split) of the Company, in accordance with the conversion notice submitted by the noteholder. Pursuant to the note agreement, the number of shares that the note converted into was based on the note balance plus accrued interest divided by $5,000,000 times the fully diluted equity of the company, excluding convertible securities issued for capital raising purposes. There was no gain or loss due to conversion, being within the terms of the agreement.
On August 9, 2021, the company entered into a convertible loan agreement with an unrelated party to loan up to $195,000 at 9% interest, with a principal balance of $72,000, as of December 31, 2021. This loan included an original issuance discount of 5%, and included 195,000 Warrants at an exercise price of $1, exercisable for 5 years from the issue date on the face of the Warrant. The noteholder has the right to convert the principal and interest into common shares of the Company. The maturity date of the loan was February 9, 2022. During the year ended December 31, 2022, another $123,000 principal with an additional $6,150 original issuance discount, was loaned to the Company. In May 2022, the Company and the note holder agreed to cancel and void previous warrants and entered into a new agreement for 225,000 warrants with an exercise price of $2.50. As of December 31, 2022, the loan was outstanding with a principal balance of $195,000, accrued interest of $35,078, amortization of debt discount of $8,393, and unamortized debt discount of $0. The warrants discussed above were initially discounted against the notes, subsequent to year end December 31, 2021, they were deemed voided and new warrants in accordance with the new terms were issued. We assessed the differences in fair value and determined that they were de minimis and expensed the full value of the new warrants. During the year ended December 31, 2022 the Company recorded an expense of $64,978.
On December 20, 2021, the company entered into a loan agreement with an unrelated party, with a principal balance of $25,000 at 6% interest. The maturity date of the loan was December 19, 2022. During the year ended December 31, 2022, the note principal was increased by $2,778 representing a 10% original issue discount pursuant to the enhanced terms mentioned below. As of December 31, 2022, the loan remained outstanding had accrued interest of $2,301. The loan was converted to common stock in February 2023 in connection with the Company IPO. Initially, the loan was estimated to be issued with 355,114 warrants. Subsequent to the entry into the December 20, 2021 the loan agreement, the Company enhanced the terms of the Bridge Note Offering under which the loan was closed and in April 2022 closed on the sale of approximately $1M in face value of convertible bridge notes, as described in footnote 13. Pursuant to the enhanced terms, the warrants will not be issued until the note converts.
On April 11, 2022, the Company entered into an Exclusive placement agent and/or underwriter agreement with WallachBeth Capital LLC in connection with a proposed private and/or public offerings by the Company. As discussed in Footnote 2, a significant component of the Company’s plan to secure capital is the intention of the Company to seek to be listed on a national exchange through an initial public offering (“IPO”) of its common stock. WallachBeth was engaged in this regard and on April 28, 2022, the Company received net proceeds of approximately $775,000 from the sale of Convertible Bridge Notes and Warrants to several institutional investors as well as several individual accredited investors. In connection with the April 28th note sale, the Company paid approximately $91,560 in fees and expenses. In addition to the money received on April 28th, the Company also received $100,000 from the sale of a Convertible Bridge Note and Warrants to a related party earlier in April. In September 2022, the Company sold one additional bridge note to an unrelated party, with a principal balance of $27,779. The Convertible Bridge Notes were issued with a 10% original issue discount and are convertible at the IPO at a 20% discount to the IPO price. The purchasers will also be issued a warrant for each share of common stock issued upon conversion of the Note at a price equal to 110% of the IPO price or, if the Company fails to complete the IPO before October 22, 2022, 90% of the IPO price. The Convertible Bridge Notes maturity date was October 31, 2022. The Company has amended the Convertible Bridge Notes to extend the maturity date until December 31, 2022. The Company has filed an S-1 Registration Statement and conducted an IPO in February 2023. All of the Convertible Bridge Notes and accrued interest through November, 30, 2022 were converted at the IPO. Pursuant to further amendments to the notes, the maturity date was extended, interest accrued after November 30, 2022 though conversion will be paid to the holders in cash and the conversion right was revised to be equal to a $25 million dollar Company valuation, or $4.27, which was also established as the warrant exercise price.
As of December 31, 2022, the table below reflects the balances of the Convertible Bridge Notes sold pursuant April 11, 2022 agreement with WallachBeth. All notes are mandatorily converted at the IPO at the conversion ratio noted above and the purchasers will also be issued a warrant for each share of common stock issued upon conversion with an exercise price set by the exchange ratio. Due to the IPO price not yet being probable at year end, no current accounting for these warrants has been journalized.
| F-13 |
| Note | Purchase | Principal | Original Issue | Accrued | ||||||||||||
| Date | Price | Balance | Discount | Interest | ||||||||||||
| 4/28/2022 | $ | 250,000 | $ | 277,778 | $ | 27,778 | $ | 17,083 | ||||||||
| 4/28/2022 | $ | 250,000 | $ | 277,778 | $ | 27,778 | $ | 17,083 | ||||||||
| 4/28/2022 | $ | 250,000 | $ | 277,778 | $ | 27,778 | $ | 17,083 | ||||||||
| 4/28/2022 | $ | 25,000 | $ | 27,778 | $ | 2,778 | $ | 1,708 | ||||||||
| 4/28/2022 | $ | 28,000 | $ | 31,111 | $ | 3,111 | $ | 1,913 | ||||||||
| 4/28/2022 | $ | 28,000 | $ | 31,111 | $ | 3,111 | $ | 1,913 | ||||||||
| 4/28/2022 | $ | 35,000 | $ | 38,889 | $ | 3,889 | $ | 2,392 | ||||||||
| 12/20/2021* | $ | 25,000 | $ | 27,778 | $ | 2,778 | $ | 2,301 | ||||||||
| 4/13/2022* | $ | 100,000 | $ | 111,111 | $ | 11,111 | $ | 7,111 | ||||||||
| 9/9/2022 | $ | 25,000 | $ | 27,778 | $ | 2,778 | $ | 1,088 | ||||||||
| Total | $ | 1,016,000 | $ | 1,128,889 | $ | 112,889 | $ | 69,675 | ||||||||
| * | Notes sold by Company prior to the April 28, 2022 closing |
In August 2020, the FASB issued ASU 2020-06, Debt - Debt with Conversion and Other Options (Subtopic 470- 20) and Derivatives and Hedging - Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”), which simplifies the accounting for certain financial instruments with characteristics of liabilities and equity. The Company specified that an entity should adopt the guidance as of the beginning of its annual fiscal year. After adoption of ASU 2020-06, if the equity securities underlying the conversion option are not readily convertible to cash, and the conversion option requires gross physical settlement of the underlying shares, the embedded conversion option may not meet the net settlement criterion, and therefore would not meet the definition of a derivative. Considering that the Common shares of the Company were not publicly traded as of December 31, 2022, the convertible options are not considered to be readily convertible to cash. In addition, the beneficial conversion feature was eliminated under ASU 2020-06. Therefore, no derivative liabilities will be triggered from these convertible notes. All conversions are contingent upon an effective IPO, which had not yet been considered probable.
NOTE 8 - CONVERTIBLE NOTES PAYABLE RELATED PARTY
Convertible Notes Payable Related Party
On July 8, 2021, the company entered into a Simple Agreement for Future Equity (SAFE), with a related party, with an amount of $150,000, with 0% interest. Under the SAFE agreement, if there is an Equity Financing before the termination of this SAFE, on the initial closing of such Equity Financing, this SAFE will automatically convert into the number of shares of SAFE Preferred Stock equal to the Purchase Amount divided by the Conversion Price, which means either: (1) the Safe Price (the price per share equal to the Post-Money Valuation Cap divided by the Company Capitalization) or (2) the Discount Price (the price per share of the Standard Preferred Stock sold in the Equity Financing multiplied by the Discount Rate), whichever calculation results in a greater number of shares of Safe Preferred Stock
If there is a Liquidity Event before the termination of this SAFE, this SAFE will automatically be entitled (subject to the liquidation priority set forth in Section 1(d) below) to receive a portion of Proceeds, due and payable to the Investor immediately prior to, or concurrent with, the consummation of such Liquidity Event, equal to the greater of (i) the Purchase Amount (the “Cash-Out Amount”) or (ii) the amount payable on the number of shares of Common Stock equal to the Purchase Amount divided by the Liquidity Price (the “Conversion Amount”). If any of the Company’s securityholders are given a choice as to the form and amount of Proceeds to be received in a Liquidity Event, the Investor will be given the same choice, provided that the Investor may not choose to receive a form of consideration that the Investor would be ineligible to receive as a result of the Investor’s failure to satisfy any requirement or limitation generally applicable to the Company’s securityholders, or under any applicable laws.
This SAFE will automatically terminate (without relieving the Company of any obligations arising from a prior breach of or non-compliance with this SAFE) immediately following the earliest to occur of: (i) the issuance of Capital Stock to the Investor pursuant to the automatic conversion of this SAFE under agreement; or (ii) the payment, or setting aside for payment, of amounts due the Investor pursuant to the agreement.
| F-14 |
As of December 31, 2022 and 2021, the $150,000 received from SAFE was recorded at 6% imputed interest. The maturity date of the loan is defined by the SAFE agreement as discussed above.
On August 19, 2021, the company entered into a convertible loan agreement with a related party, with a principal balance of $99,900 at 9% interest. The noteholder has the right to convert the principal and interest into common shares of the Company. This loan included an original issuance discount of 5% and included 99,900 Warrants at an exercise price of $1, exercisable for 5 years from the issue date on the face of the Warrant. The maturity date of the loan was February 19, 2022. In May 2022, the Company and the note holder agreed to cancel and void previous warrants and entered into a new agreement for 115,185 warrants with an exercise price of $2.50. As of December 31, 2022, the $99,900 principal and the $4,950 overpayment of the note remained outstanding and had accrued interest of $12,463.53. The warrants discussed above were initially discounted against the notes, subsequent to year end December 31, 2021, they were deemed voided and new warrants in accordance with the new terms were issued. We assessed the differences in fair value and determined that they were de minimis and expensed the full value of the new warrants.
The SAFE and the convertible loan agreement with accrued interest converted to common stock at the IPO.
The Company specified that an entity should adopt ASU 2020-06 as of the beginning of its annual fiscal year. After adoption of ASU 2020-06, no derivative liabilities will be triggered from these convertible notes. See Note 7 for details.
NOTE 9 -RELATED PARTY
Related Party
During the year-ended December 31, 2021, there were 57,143 shares of common stock (post reverse stock split) issued to CFO Dane Saglio, for services rendered.
As of December 31, 2022 and 2021, the accrued salary for related parties was $566,916 and $285,666, respectively. The increase reflects salaries accrued for employees, but not paid in the year ended December 31, 2022.
As of December 31, 2022, the Company accrued consulting fees to related parties of $90,000 for services provided to the Company.
During the year ended December 31, 2021, the Company issued options totaling 29,286 shares of common stock (post reverse stock split) to related party for services rendered. The options have an original life of ten years and vest at different rates over as much as 24 months. During the year ended December 31, 2022, the Company did not issue any options and recognized $1,803 of stock-based compensation related to outstanding stock options.
NOTE 10- SHAREHOLDER’S DEFICT
Stockholder’s Equity
Preferred Stock
The Company has 10,000,000 shares of preferred stock authorized at a par value of $0.00001. As of December 31, 2021, there were no preferred shares issued. On October 5, 2022, the Company entered into an exchange agreement with the Investor whereby all of his common stock, 734,492 shares of commons stock (post reverse stock split), were exchanged into 73,449 shares of Series A Convertible Preferred Stock (post reverse stock split). Per the agreement the exchange was based on a 1 Series A Convertible Preferred Stock for each 10 shares of common stock. Each holder of Series A Preferred Stock may, from time to time, convert any or all of such holder’s shares of Series A Preferred Stock into fully paid and nonassessable shares of Common Stock in an amount equal to ten shares of common stock for each one share of Series A Preferred Stock surrendered. The Series A Preferred Stock is the economic equivalent of the common stock but has no voting rights and is subject to a blocker which prohibits the conversion into common stock if it would result in the Investor owning more than 4.99% of the Company’s outstanding common stock at such time. The Company evaluated the terms of the exchange and determined there would be no significant change in fair value and therefore no accounting entry recorded as a result of the exchange. The value of the Series A Preferred Stock was determined to be $315,000 which is the Investor’s basis in the common stock that was exchanged.
Common Stock
In June of 2020, BullFrog AI Holdings, Inc. acquired BullFrog AI, Inc. via a 1:1 share exchange. Immediately prior to the share exchange, each authorized common share of BullFrog AI, Inc. was split into 25 shares of common stock. Share amounts in our financial statements for December 31, 2022 and 2021, have been adjusted to reflect this forward share split and shares exchange. All of our operations are currently conducted through BullFrog AI Holdings, Inc. BullFrog AI, Inc., is a wholly owned subsidiary, has the sole purpose of housing and protecting all of the organization’s intellectual property. BullFrog AI Management, LLC is a wholly owned subsidiary that handles all HR and payroll activities.
The Company has 100,000,000 shares of common stock authorized at a par value of $0.00001. During year ended December 31, 2022, 734,492 shares of common stock (post reverse stock split) were exchanged for preferred shares as noted above, 205,984 shares of common stock (post reverse stock split) were issued for conversion of principal and interest of $226,138 by a noteholder, 112,225 shares of common stock (post reverse stock split) were canceled as the change in number of shares issued as part of the cancellation of the prior agreements and new agreements with advisors, and 38,879 shares of common stock (post reverse stock split) were issued under a license agreement and valued at $189,828, see Note 12 for further discussion. As of December 31, 2022 and 2021, there are 4,021,935 and 4,622,789, shares of common stock (post reverse stock split) outstanding, respectively.
| F-15 |
After the Company signed two licenses for two drug programs from universities in the first half of 2022 it engaged an independent valuation firm to perform an Enterprise-Equity valuation. The results of this engagement resulted in an increase in the value per share of common stock used in the Black Scholes option pricing model employed to value the Company’s equity grants and warrant issuances.
Our Board of Directors and stockholders approved an amendment to our Certificate of Incorporation to effect a 1-for-7 reverse stock split of our common stock in connection with the offering, subsequent to the year ended December 31, 2022. As a result of the reverse stock split, every 7 shares of our outstanding common stock will be combined and reclassified into one share of our common stock. Unless otherwise noted, the share and per share information in this Form 10-K filing reflects, other than in our historical financial statements and the notes thereto, a proposed reverse stock split of the outstanding common stock of the Company at an assumed 1-for-7 ratio.
Stock Options
During the first quarter of 2022, 399,354 shares of options (post reverse stock split) were forfeited due to the termination of employment.
During the year ended December 31, 2021, the Company granted a total of 29,286 shares of options (post reverse stock split) to employees of the Company for services rendered. The options have an original life of ten years and vest at different rates over as much as 48 months. During the years ended December 31, 2021, the Company vested 1,310 of these options (post reverse stock split) and recognized $157 of stock-based compensation related to outstanding stock options. During the year ended December 31, 2022, 16,601 shares of these options (post reverse stock split) were vested and $2,010 stock-based compensation was recognized.
The following tables summarizes the stock options (post reverse stock split) activity for the years ended December 31, 2022 and 2021:
Schedule of Stock Options Activity
| Granted and outstanding, December 31, 2020 | 884,821 | |||
| Granted during 2021 | 29,286 | |||
| Exercised | - | |||
| Forfeited | - | |||
| Expired during 2021 | (445,536 | ) | ||
| Granted and outstanding, December 31, 2021 | 468,571 | |||
| Granted during 2022 | - | |||
| Exercised | - | |||
| Forfeited | (399,354 | ) | ||
| Expired during 2022 | - | |||
| Granted and outstanding, December 31, 2022 | 69,217 |
Schedule of Vested and Outstanding Options
| Options | Intrinsic Value of Vested Options | Weight Averaged exercise Price | ||||||||||
| Vested and outstanding, December 31, 2020 | 104,795 | 12,706 | 3.36 | |||||||||
| Granted and vested during 2021 | 1,310 | 157 | 2.66 | |||||||||
| Exercised | - | - | - | |||||||||
| Forfeited | - | - | - | |||||||||
| Expired | (66,524 | ) | (7,922 | ) | (3.36 | ) | ||||||
| Vested and outstanding, December 31, 2021 | 39,581 | 4,941 | 3.36 | |||||||||
| Granted and vested during 2022 | 16,661 | 2,010 | 2.73 | |||||||||
| Exercised | - | - | - | |||||||||
| Forfeited | - | - | - | |||||||||
| Expired | - | - | - | |||||||||
| Vested and outstanding, December 31, 2022 | 56,242 | 6,951 | 3.15 | |||||||||
As of December 31, 2022 and 2021, 16,661 and 1,310 options (post reverse stock split) vested, respectively, 0 and 66,524 (post reverse stock split) options expired and the outstanding stock options have a weighted average remaining life of 7.08 and 7.38 years, respectively.
As of December 31, 2022 and 2021, the fair value of options vested and outstanding was $ 6,951 and $4,941, respectively. The aggregate fair value of the options measured during the year ended December 31, 2022 and 2021 was calculated using the Black-Scholes option pricing model based on the following assumption:
Schedule of Black Scholes Option Pricing Model
| December 31, 2022 | December 31, 2021 | |||||||
| Fair Value of Common Stock on measurement date | $ | 4.76 | $ | 0.308 | ||||
| Risk free interest rate | From 0.79% to 3.01 | % | From 1.26% to 1.33 | % | ||||
| Volatility | 89 | % | 93 | % | ||||
| Dividend Yield | 0 | % | 0 | % | ||||
| Expected Term | 4-10 | 10 | ||||||
| (1) | The risk-free interest rate was determined by management using the market yield on U.S. Treasury securities with comparable terms as of the measurement date. | |
| (2) | The trading volatility was determined by calculating the volatility of the Company’s peer group. | |
| (3) | The Company does not expect to pay a dividend in the foreseeable future. |
| F-16 |
Warrants
During the year ended December 31, 2022, the Company granted a total of 123,660 warrants (post reverse stock split). The warrants have an original life of four to ten years and vest immediately and over 12 months. During the year ended December 31, 2022, 174,105 shares of warrants (post reverse stock split) were vested and amended with an intrinsic value of $337,269, 51,941 shares of warrants (post reverse stock split) were reclassified with an intrinsic value of $11,097, and 42,057 shares of warrants (post reverse stock split) with an intrinsic value of $1,883 were forfeited.
During the year ended December 31, 2021, the Company granted a total of 431,659 warrants (post reverse stock split). Of this amount 200,000 warrants (post reverse stock split), with a fair value of $12,462, were granted to advisors related to the Company’s IPO objective. The warrants have an original life of five years and vest 30 days before the intended IPO. During the year ended December 31, 2021, 0 shares of these warrants were vested. As of June 30, 2022, the warrants for 200,000 shares (post reverse stock split) were cancelled and voided per agreement of the warrant holder and the Company. There was no gain or loss due to cancellation. In 2021, 138,929 warrants (post reverse stock split), with a fair value of $28,683, were issued for services rendered. The warrants have an original life of ten years and vest at different rates over as much as 36 months.
During the year ended December 31, 2021, the Company issued 92,859 warrants (post reverse stock split) with a fair value of $12,980, in connection with convertible bridge debt agreements with multiple parties including a related party. The warrants had an original life of five years. During the period ending June 30, 2022, the Company determined that 50,735 warrants (post reverse stock split), with a fair value of $11,097, should not have been issued as further described in footnote 8. The fair value was reclassified to Additional Paid in Capital. As discussed in Note 8 in May 2022, the Company and the note holders agreed to cancel and void the previous 99,000 warrants (post reverse stock split) and entered into a new agreement for 115,185 (post reverse stock split) and the exercise price increased to $2.50 from $1, with a fair value of $15,412. As discussed in Note 8 in May 2022, the Company and the note holders agreed to cancel and void the previous 195,000 (post reverse stock split) warrants and entered into a new agreement for 225,000 warrants (post reverse stock split) with an exercise price of $2.50, with a fair value of $64,978.
The 92,859 warrants (post reverse stock split) discussed above were initially discounted against the notes, subsequent to year end December 31, 2021, they were deemed voided and these individuals were or will be issued new warrants in accordance with the new terms as stated above. We assessed the differences in fair values and determined the values were de minimis and expensed the full value of the new warrants.
The following tables summarize the warrant activity (post reverse stock split) for the year ended December 31, 2022 and 2021,
Schedule of Stock Warrant Activity
| Warrants | ||||
| Granted and outstanding, December 31, 2020 | 495,714 | |||
| Granted during 2021 | 431,659 | |||
| Exercised | - | |||
| Forfeited | - | |||
| Expired during 2021 | - | |||
| Granted and outstanding, December 31, 2021 | 927,373 | |||
| Granted during 2022 | 123,660 | |||
| Exercised | - | |||
| Forfeited | (298,088 | ) | ||
| Expired during 2022 | - | |||
| Granted and outstanding, December 31, 2022 | 752,945 | |||
Schedule of Vested And Outstanding Warrants
| Warrants | Intrinsic Value of Warrants | Weight Averaged exercise Price | ||||||||||
| Vested and outstanding, December 31, 2020 | 479,940 | 127,480 | 0.98 | |||||||||
| Granted and Vested 2021 | 137,552 | 22,208 | 3.15 | |||||||||
| Exercised | - | - | - | |||||||||
| Forfeited | - | - | - | |||||||||
| Expired | - | - | - | |||||||||
| Vested and outstanding, December 31, 2021 | 617,492 | 149,688 | 2.80 | |||||||||
| Granted and Vested 2022 | 174,105 | 337,263 | 3.15 | |||||||||
| Exercised | - | - | - | |||||||||
| Forfeited | (94,665 | ) | (12,980 | ) | - | |||||||
| Expired | - | - | - | |||||||||
| Vested and outstanding, December 31, 2022 | 696,932 | 473,971 | 1.96 | |||||||||
| F-17 |
As of December 31, 2022, 752,945 warrants (post reverse stock split) are outstanding, and 696,932 warrants (post reverse stock split) vested, and the vested stock warrants have a weighted average remaining life of 7.13 years.
For the year ended December 31, 2022, the aggregate fair value of warrants vested was $324,283. The aggregate fair value of the warrants measured during the year ended December 31, 2022 was calculated using the Black-Scholes option pricing model and recorded as stock-based compensation.
For the year ended December 31, 2021, 927,516 warrants (post reverse stock split) are outstanding, 617,492 warrants (post reverse stock split) are vested with an intrinsic value of $22,208, and the vested stock warrants have a weighted average remaining life of 7.73 years.
As of December 31, 2021, the aggregate fair value of warrants vested was $149,688. The aggregate fair value of the warrants measured during the year-ended December 31, 2021 was calculated using the Black-Scholes option pricing model.
The number of warrants related to the Convertible Bridge Notes discussed Note 7 is not yet determinable, given some of the terms discussed in Note 8 have not been completed. Therefore, the warrants to be issued are not accounted for in our warrants outstanding. Due to the IPO price not being completed at December 31, 2022, no current accounting for these warrants has been journalized.
Schedule of Black Scholes Option Pricing Model
| December 31, 2022 | December 31, 2021 | |||||||
| Fair Value of Common Stock on measurement date | $ | 4.76 | $ | 0.308 | ||||
| Risk free interest rate | From 1.86% to 1.97 | % | From 0.78% to 1.63 | % | ||||
| Volatility | 89 | % | 93 | % | ||||
| Dividend Yield | 0 | % | 0 | % | ||||
| Expected Term | 10 years | 5-10 years | ||||||
| (1) | The risk-free interest rate was determined by management using the market yield on U.S. Treasury securities with comparable terms as of the measurement date. | |
| (2) | The trading volatility was determined by calculating the volatility of the Company’s peer group. | |
| (3) | The Company does not expect to pay a dividend in the foreseeable future. | |
| (4) | After the Company signed two licenses for two drug programs from universities in the first half of 2022 it engaged an independent valuation firm to perform an Enterprise-Equity valuation. The results of this engagement resulted in an increase in the value per share of common stock used in the Black Scholes option pricing model employed to value the Company’s equity grants and warrant issuances for all 2022 grant date stock prices. |
Schedule of Outstanding Warrants
NOTE 11 - INCOME TAXES
Income Taxes
As of December 31, 2022, the Company has available for federal income tax purposes a net operating loss carry forward of approximately $4,399,055, that do not expire, that may be used to offset future taxable income, but could be limited under Section 382. The Company has provided a valuation reserve against the full amount of the net operating loss benefit, since in the opinion of management based upon the earnings history of the Company; it is more likely than not that the benefits will not be realized. Due to possible significant changes in the Company’s ownership, the future use of its existing net operating losses may be limited. All or portion of the remaining valuation allowance may be reduced in future years based on an assessment of earnings sufficient to fully utilize these potential tax benefits.
We have adopted the provisions of ASC 740-10-25, which provides recognition criteria and a related measurement model for uncertain tax positions taken or expected to be taken in income tax returns. ASC 740-10-25 requires that a position taken or expected to be taken in a tax return be recognized in the financial statements when it is more likely than not that the position would be sustained upon examination by tax authorities.
Tax position that meets the more likely than not threshold is then measured using a probability weighted approach recognizing the largest amount of tax benefit that is greater than 50% likely of being realized upon ultimate settlement. The Company had no tax positions relating to open income tax returns that were considered to be uncertain. We file income tax returns in the U.S. and in the state of California and Utah with varying statutes of limitations.
The Company’s deferred taxes as of December 31, 2022 and 2021 consist of the following:
Schedule of Deferred Taxes
| 2022 | 2021 | |||||||
| Non-Current deferred tax asset: | ||||||||
| Net operating loss carryforwards | $ | 924,000 | $ | 339,000 | ||||
| Valuation allowance | (924,000 | ) | (339,000 | ) | ||||
| Net non-current deferred tax asset | $ | - | $ | - | ||||
| F-18 |
NOTE 12 - MATERIAL AGREEMENTS
Material Agreements
JHU-APL Technology License
On February 7, 2018, the Company entered into an exclusive, world-wide, royalty-bearing license from JHU-APL for the technology. The license covers three (3) issued patents, 1 new provisional patent application, non-patent rights to proprietary libraries of algorithms and other trade secrets, the license also includes modifications and improvements. In October of 2021, the Company executed an Amendment to the original license which represents improvements and new advanced analytics capabilities. In consideration of the rights granted to the Company under the License Agreement JHU received a warrant equal to five (5%) percent of the then fully diluted equity base of the Company, which shall be diluted following the closing of this offering. Under the terms of the License Agreement, JHU will be entitled to eight (8%) percent royalty on net sales for the services provided by the Company in which the JHU licensed technology was utilized, as well as fifty (50%) percent of all sublicense revenues received by the Company. In addition, the Company is required to pay JHU an annual maintenance fee of $1,500. Minimum annual royalty payments are $20,000 for 2022, $80,000 for 2023, and $300,000 for 2024 and beyond, if cumulative annual royalty payments do not reach these levels, the amount due to JHU to reach the annual minimum is due by January 31st of the following year. Failure to make annual royalty payments is considered a material breach under the agreement and upon notice from JHU of a material breech, the Company shall have 60 days to cure the material breech. On July 8, 2022, the company entered into an exclusive, world-wide, royalty-bearing license from JHU-APL for the additional technology developed to enhance the bfLEAP™ platform. The new license provides additional intellectual property rights including patents, copyrights and knowhow to be utilized under the Company’s bfLEAP™ analytical AI/ML platform. This license supersedes the previous license. In consideration of the new license, the Company issued 279,159 shares of common stock. (see note 10) Under the terms of the new License Agreement, JHU will be entitled to eight (8%) percent of net sales for the services provided by the Company to other parties and 3% for internally development drug projects in which the JHU license was utilized. The new license also contains tiered sub licensing fees that start at 50% and reduce to 25% based on revenues. In addition, under the new license agreement, the minimum annual royalty payments are $30,000 for 2022, $80,000 for 2023, and $300,000 for 2024 and beyond. As of December 31, 2022, we have accrued, $30,000 of the 2022 minimum annual royalty payments. See Note 10 for details on common shares and warrants issued related to this agreement.
George Washington University - Beta2-spectrin siRNA License
On January 14, 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from George Washington University (GWU) for rights to use siRNA targeting Beta2-spectrin in the treatment of human diseases, including hepatocellular carcinoma (HCC). The license covers methods claimed in three US and worldwide patent applications, and also includes use of this approach for treatment of obesity, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis.
In consideration of the rights granted to the Company under the License Agreement GWU received a $20,000 License Initiation Fee. Under the terms of the License Agreement, GWU will be entitled to a three percent (3%) royalty on net sales subject to quarterly minimums once the first sale has occurred subsequent to regulatory approval, as well sublicense or assignment fees in the event the Company sublicenses or assigns their rights to use the technology. The Company will also reimburse GWU for previously incurred and ongoing patent costs. The Sublicense and Assignment fee amounts decline as the Company advances the clinical development of the licensed technology. The license agreement also contains milestone payments for clinical development through the approval of an NDA and commercialization. As of December 31, 2022, there has been no accrual for royalties, since we have not begun revenue. The Company assessed whether the license should be capitalized and determined that the licensed program is early stage and therefore the Company expensed the license fee and will expense development costs until commercial viability is likely.
Johns Hopkins University - Mebendazole License
On February 22, 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from Johns Hopkins University (JHU) for the use of an improved formulation of Mebendazole for the treatment of any human cancer or neoplastic disease. This formulation shows potent activity in animal models of different types of cancer and has been evaluated in a Phase I clinical trial in patients with high-grade glioma (NCT01729260). The trial, an open-label dose-escalation study, assessed the safety and efficacy of the improved formulation with adjuvant temozolomide in 24 patients with newly diagnosed gliomas. Investigators observed no dose-limiting toxicity in patients receiving all but the highest tested dose (200mg/kg/day). Four of the 15 patients receiving the maximum tested dose of 200mg/kg/day experienced dose-limiting toxicity, all of which were reversed by decreasing or eliminating the dose given. There were no serious adverse events attributed to mebendazole at any dose during the trial. 41.7% of patients who received mebendazole were alive at two years after enrollment, and 25% were alive at four years (Gallia et al., 2021).
| F-19 |
The license covers six (6) issued patents and one (1) pending application. In consideration of the rights granted to the Company under the License Agreement JHU will receive a staggered Upfront License Fee of $250,000. The Company will also reimburse JHU for previously incurred and ongoing patent costs. Under the terms of the License Agreement, JHU will be entitled to three- and one-half percent (3.5%) royalty on net sales by the Company. In addition, the Company is required to pay JHU minimum annual royalty payments of $5,000 for 2023, $10,000 for 2024, $20,000 for 2025, $30,000 for 2026 and $50,000 for 2027 and each year after until the first commercial sale after which the annual minimum royalty shall be $250,000. The license agreement also contains milestone payments for clinical development steps through the approval of an NDA and commercialization. The license covers six (6) issued patents and one (1) pending application. In consideration of the rights granted to the Company under the License Agreement JHU will receive a staggered Upfront License Fee of $250,000. The initial payment for $50,000 was paid and the remaining balance is deferred until the earlier of; we complete the IPO, raise $10 million in financing or until 9 months from the effective date of the license. As of December 31, 2022, the balance of accrued expense related to this license agreement was $242,671. The Company assessed whether the license should be capitalized and determined that the licensed program is early stage and therefore the Company expensed the license fee and will expense development costs until commercial viability is likely.
Johns Hopkins University - Prodrug License
On October 13, 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from Johns Hopkins University (JHU) and the Institute of Organic Chemistry and Biochemistry (IOCB) of the Czech Academy of Sciences for rights to commercialize N-substituted prodrugs of mebendazole that demonstrate improved solubility and bioavailability. The license covers prodrug compositions and use for treating disease as claimed in multiple US and worldwide patent applications. In consideration for the rights granted to the Company under the License Agreement JHU and IOCB will receive a staggered upfront license fee of $100,000. The Company will also reimburse JHU and IOCB for previously incurred patent costs. Under the terms of the License Agreement, JHU and IOCB will be entitled to four percent (4.0%) royalty on net sales by the Company. In addition, the Company is required to pay JHU and IOCB minimum annual royalty payments of $5,000 for 2027, $10,000 for 2028, $20,000 for 2029, $30,000 for 2030 and $50,000 for 2031 and each year after until the first commercial sale after which the annual minimum royalty shall be $150,000. The license agreement also contains milestone payments for patent grants, clinical development steps through the approval of an NDA and commercialization. As of December 31, 2022, the balance of accrued expense related to this license agreement was $133,238. The Company assessed whether the license should be capitalized and determined that the licensed program is early stage and therefore the Company expensed the license fee and will expense development costs until commercial viability is likely.
NOTE 13 - COMMITMENTS AND CONTINGENCIES
Commitments and Contingencies
The Company follows ASC 450, Contingencies, which requires the Company to assess the likelihood that a loss will be incurred from the occurrence or non-occurrence of one or more future events. Such assessment inherently involves an exercise of judgment. In assessing possible loss contingencies from legal proceedings or unasserted claims, the Company evaluates the perceived merits of such proceedings or claims, and of the relief sought or expected to be sought.
If the assessment of a contingency indicates that it is probable that a material loss will be incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s financial statements. If the assessment indicates that a potentially material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, and an estimate of the range of possible losses, if determinable and material, would be disclosed. Loss contingencies considered remote are generally not disclosed unless they involve guarantees, in which case the guarantees would be disclosed.
While not assured, management does not believe, based upon information available at this time, that a loss contingency will have material adverse effect on the Company’s financial position, results of operations or cash flows.
NOTE 14 - SUBSEQUENT EVENTS
Subsequent Events
On February 14, 2023, the Company conducted its initial public offering of 1,297,318 units (each, a “Unit,” collectively, the “Units”) at a price of $6.50 per unit for a total of approximately $8.4 million of gross proceeds to the Company. Each Unit consists of one share of the Company’s common stock, one tradeable warrant (each, a “Tradeable Warrant,” collectively, the “Tradeable Warrants”) to purchase one share of common stock at an exercise price of $7.80 per share, and one non-tradeable warrant (each, a “Non-tradeable Warrant,” collectively, the “Non-tradeable Warrants”; together with the Tradeable Warrants, each, a “Warrant,” collectively, the “Warrants”) to purchase one share of the Company’s common stock at an exercise price of $8.125. The offering closed on February 16, 2023.
In connection with the offering, the Company common shares were subject to a 1-7 reverse stock split - 1 share of new common for 7 shares then outstanding common stock. Also, in connection with the IPO a SAFE and convertible loan agreement held by a related party converted into 55,787 shares of post reverse common stock. Additionally, all outstanding Convertible Bridge Notes and accrued interest through November 30, 2022 were converted into 276,289 shares common stock and 276,289 warrants to purchase common stock were issued to the Convertible Bridge Note holders at conversion. The Bridge Note conversions and the warrant exercise pricing was determined using a $25 million dollar company valuation immediately before the IPO.
Between April 5 and April 13, 2023, the holders of warrants exercised 436,533 warrants for common shares at various exercise prices and the Company received proceeds of approximately $1,495,000.
| F-20 |
Bullfrog AI Holdings, Inc.
Condensed Consolidated Balance Sheets
As of September 30, 2023 and December 31, 2022
(Unaudited)
| September 30, 2023 | December 31, 2022 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 3,856,037 | $ | 57,670 | ||||
| Prepaid expenses | 352,433 | 15,000 | ||||||
| Total current assets | 4,208,470 | 72,670 | ||||||
| Property and equipment, net | 6,406 | 7,699 | ||||||
| Total assets | $ | 4,214,876 | $ | 80,369 | ||||
| Liabilities and Stockholders’ Deficit | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 110,503 | $ | 543,993 | ||||
| Accrued expenses | 122,257 | 982,988 | ||||||
| Deferred revenue | - | 32,000 | ||||||
| Short term insurance financing | 213,290 | - | ||||||
| Convertible notes | - | 1,323,890 | ||||||
| Convertible notes - related party | - | 254,850 | ||||||
| Total current liabilities | 446,050 | 3,137,721 | ||||||
| Stockholders’ deficit: | ||||||||
| Series A Convertible Preferred stock, $0.00001 par value, 5,500,000 shares authorized; 73,449 shares issued and outstanding, as of September 30, 2023 and December 31, 2022. | 1 | 1 | ||||||
| Common stock, $0.00001 par value, 100,000,000 shares authorized; 6,094,644 and 4,021,935 shares issued and outstanding as of September 30, 2023 and December 31, 2022, respectively. | 61 | 40 | ||||||
| Additional paid-in capital | 12,226,742 | 1,341,662 | ||||||
| Accumulated deficit | (8,457,978 | ) | (4,399,055 | ) | ||||
| Total stockholders’ deficit | 3,768,826 | (3,057,352 | ) | |||||
| Total liabilities and stockholders’ deficit | $ | 4,214,876 | $ | 80,369 | ||||
See accompanying notes to unaudited condensed consolidated financial statements.
| F-21 |
Bullfrog AI Holdings, Inc.
Condensed Consolidated Statements of Operations
For the Three Months and Nine Months Ended September 30, 2023 and 2022
(Unaudited)
| Three Months Ended | Nine Months Ended | |||||||||||||||
| September 30, | September 30, | |||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||
| Revenue: | ||||||||||||||||
| Revenue, net | $ | 65,000 | $ | - | $ | 65,000 | $ | - | ||||||||
| Total revenue | 65,000 | - | 65,000 | - | ||||||||||||
| Cost of goods sold: | ||||||||||||||||
| Cost of goods sold | 5,200 | - | 5,200 | - | ||||||||||||
| Total cost of goods sold | 5,200 | - | 5,200 | - | ||||||||||||
| Gross profit | 59,800 | - | 59,800 | - | ||||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | 380,015 | 39,421 | 1,023,619 | 448,375 | ||||||||||||
| General and administrative | 983,929 | 601,131 | 3,067,940 | 1,424,383 | ||||||||||||
| Total operating expenses | 1,363,944 | 640,552 | 4,091,559 | 1,872,758 | ||||||||||||
| Loss from operations | (1,304,144 | ) | (640,552 | ) | (4,031,759 | ) | (1,872,758 | ) | ||||||||
| Other income (expense), net | ||||||||||||||||
| Interest expense, net | (5,758 | ) | (124,159 | ) | (76,880 | ) | (234,668 | ) | ||||||||
| Loss on conversion of notes | - | - | (92,959 | ) | - | |||||||||||
| Other (expense) income, net | 56,924 | 18 | 142,675 | 457 | ||||||||||||
| Total other income (expense), net | 51,166 | (124,141 | ) | (27,164 | ) | (234,211 | ) | |||||||||
| Net loss | $ | (1,252,978 | ) | $ | (764,693 | ) | $ | (4,058,923 | ) | $ | (2,106,969 | ) | ||||
| Net loss per common share attributable to common stockholders - basic and diluted | $ | (0.21 | ) | $ | (0.16 | ) | $ | (0.72 | ) | $ | (0.45 | ) | ||||
| Weighted average number of shares outstanding - basic and diluted | 6,094,644 | 4,752,959 | 5,667,997 | 4,669,952 | ||||||||||||
See accompanying notes to unaudited condensed consolidated financial statements.
| F-22 |
Bullfrog AI Holdings, Inc.
Condensed Consolidated Statements of Changes in Stockholders’ Deficit
For the Three Months Ended September 30, 2023 and December 31, 2022
(Unaudited)
| Series A Preferred Stock | Common Stock | Additional Paid-in | Accumulated | Total Stockholders’ | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||||||||
| Balance at December 31, 2022 | 73,449 | $ | 1 | 4,021,935 | $ | 40 | $ | 1,341,662 | $ | (4,399,055 | ) | $ | (3,057,352 | ) | ||||||||||||||
| Stock-based compensation | - | - | - | - | 127,450 | - | 127,450 | |||||||||||||||||||||
| Issuance of common stock (initial public offering), net of issuance cots | - | - | 1,297,318 | 13 | 7,293,638 | - | 7,293,651 | |||||||||||||||||||||
| Issuance of common stock for services | - | - | 7,692 | 1 | 49,999 | - | 50,000 | |||||||||||||||||||||
| Conversion of convertible debt to common stock | - | - | 331,166 | 3 | 1,535,612 | - | 1,535,615 | |||||||||||||||||||||
| Net loss | - | - | - | - | - | (1,325,547 | ) | (1,325,547 | ) | |||||||||||||||||||
| Balance at March 31, 2023 | 73,449 | 1 | 5,658,111 | 57 | 10,348,361 | (5,724,602 | ) | 4,623,817 | ||||||||||||||||||||
| Stock-based compensation | - | - | 262,267 | 262,267 | ||||||||||||||||||||||||
| Issuance of common stock pursuant to warrant exercises | 436,533 | 4 | 1,494,654 | 1,494,658 | ||||||||||||||||||||||||
| Net loss | (1,480,398 | ) | (1,480,398 | ) | ||||||||||||||||||||||||
| Balance at June 30, 2023 | 73,449 | 1 | 6,094,644 | 61 | 12,105,282 | (7,205,000 | ) | 4,900,344 | ||||||||||||||||||||
| Stock-based compensation | - | - | 121,460 | 121,460 | ||||||||||||||||||||||||
| Net loss | (1,252,978 | ) | (1,252,978 | ) | ||||||||||||||||||||||||
| Balance at September 30, 2023 | 73,449 | $ | 1 | 6,094,644 | $ | 61 | $ | 12,226,742 | $ | (8,457,978 | ) | $ | 3,768,826 | |||||||||||||||
| Balance at December 31, 2021 | - | $ | - | 4,622,789 | $ | 46 | $ | 587,415 | $ | (1,596,568 | ) | (1,009,107 | ) | |||||||||||||||
| Imputed interest | - | - | - | - | 2,360 | - | 2,360 | |||||||||||||||||||||
| Stock-based compensation | - | - | - | - | 30,017 | - | 30,017 | |||||||||||||||||||||
| Reclassification of warrant | - | - | - | - | (11,097 | ) | - | (11,097 | ) | |||||||||||||||||||
| Net loss | - | - | - | - | - | (566,359 | ) | (566,359 | ) | |||||||||||||||||||
| Balance at March 31, 2022 | - | - | 4,622,789 | 46 | 608,695 | (2,162,927 | ) | (1,554,186 | ) | |||||||||||||||||||
| Imputed interest | - | - | - | - | 2,361 | - | 2,361 | |||||||||||||||||||||
| Stock-based compensation | - | - | - | - | 209,323 | - | 209,323 | |||||||||||||||||||||
| Conversion of convertible notes | - | - | 205,984 | 2 | 226,136 | - | 226,138 | |||||||||||||||||||||
| Shares cancellation | - | - | (112,225 | ) | (1 | ) | 1 | - | - | |||||||||||||||||||
| Net loss | - | - | - | - | - | (775,917 | ) | (775,917 | ) | |||||||||||||||||||
| Balance at June 30, 2022 | - | - | 4,716,548 | 47 | 1,046,516 | (2,938,844 | ) | (1,892,281 | ) | |||||||||||||||||||
| Imputed interest | - | - | - | - | 2,250 | - | 2,250 | |||||||||||||||||||||
| Stock-based compensation | - | - | - | - | 51,536 | - | 51,536 | |||||||||||||||||||||
| Shares issuance for license | - | - | 39,879 | - | 189,828 | - | 189,828 | |||||||||||||||||||||
| Net loss | - | - | - | - | - | (764,693 | ) | (764,693 | ) | |||||||||||||||||||
| Balance at September 30, 2022 | - | $ | - | 4,756,427 | $ | 47 | $ | 1,290,130 | $ | (3,703,537 | ) | $ | (2,413,360 | ) | ||||||||||||||
See accompanying notes to unaudited condensed consolidated financial statements.
| F-23 |
Bullfrog AI Holdings, Inc.
Condensed Consolidated Statements of Cash Flows
For the Nine Months Ended September 30, 2023 and 2022
(Unaudited)
| Nine Months Ended September 30, | ||||||||
| 2023 | 2022 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (4,058,923 | ) | $ | (2,106,969 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | 1,293 | 604 | ||||||
| Stock-based compensation | 511,177 | 290,876 | ||||||
| Shares issued for license | - | 189,828 | ||||||
| Shares issued for services | 50,000 | - | ||||||
| Loss on conversion of notes | 92,959 | - | ||||||
| Amortization of debt discount | 20,000 | 174,998 | ||||||
| Imputed interest | - | 6,971 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expense | (337,433 | ) | (15,000 | ) | ||||
| Accounts payable | (433,490 | ) | 51,946 | |||||
| Accrued expenses | (796,865 | ) | 409,502 | |||||
| Accrued expenses - related party | - | 104,000 | ||||||
| Deferred revenue | (32,000 | ) | 22,000 | |||||
| Net cash used in operating activities | (4,983,282 | ) | (871,244 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchases of property and equipment | - | (8,744 | ) | |||||
| Net cash used in investing activities | - | (8,744 | ) | |||||
| Cash flows from financing activities: | ||||||||
| Proceeds from issuance of common stock (initial public offering), net of issuance costs | 7,293,651 | - | ||||||
| Proceeds from exercise of warrants | 1,494,658 | - | ||||||
| Proceeds from convertible notes payable | - | 961,190 | ||||||
| Proceeds from notes payable | 100,000 | - | ||||||
| Payments of notes payable | (319,950 | ) | - | |||||
| Repayment of note payable and interest - related party | - | (49,000 | ) | |||||
| Proceeds net of payments short term insurance financing | 213,290 | - | ||||||
| Net cash provided by financing activities | 8,781,649 | 912,190 | ||||||
| Net increase in cash and cash equivalents | 3,798,367 | 32,202 | ||||||
| Cash and cash equivalents, beginning of period | 57,670 | 10,014 | ||||||
| Cash and cash equivalents, end of period | $ | 3,856,037 | $ | 42,216 | ||||
| Supplemental cash flow information: | ||||||||
| Cash paid for interest | $ | 93,916 | $ | 4,399 | ||||
| Cash paid for taxes | - | - | ||||||
| Supplemental non-cash activity | ||||||||
| Reclassification of warrant | $ | - | $ | 11,097 | ||||
| Issuance of common stock upon conversion of notes payable | $ | 1,535,615 | $ | - | ||||
| Conversion of convertible note payable | $ | - | $ | 226,138 | ||||
See accompanying notes to unaudited condensed consolidated financial statements.
| F-24 |
Bullfrog AI Holdings, Inc.
Notes to Condensed Consolidated Financial Statements (Unaudited)
1. Organization and Nature of Business
Description of the Business
Bullfrog AI Holdings, Inc. (“we”, “our” or the “Company”) was incorporated in the State of Nevada on February 6, 2020. Bullfrog AI Holdings, Inc. is the parent company of Bullfrog AI, Inc. and Bullfrog AI Management, LLC. which were incorporated in Delaware and Maryland, in 2017 and 2021, respectively. All of our operations are currently conducted through Bullfrog AI Holdings, Inc., which began operations on February 6, 2020. We are a company focused specifically on advanced AI/ML-driven analysis of complex data sets in medicine and healthcare. Our objective is to utilize our platform for precision medicine approach to drug asset enablement through external partnerships and selective internal development.
Most new therapeutics will fail at some point in preclinical or clinical development. This is the primary driver of the high cost of developing new therapeutics. A major part of the difficulty in developing new therapeutics is efficient integration of complex and highly dimensional data generated at each stage of development to de-risk subsequent stages of the development process. Artificial Intelligence and Machine Learning (AI/ML) has emerged as a digital solution to help address this problem.
We use artificial intelligence and machine learning to advance medicines for both internal and external projects. Most current AI/ML platforms still fall short in their ability to synthesize disparate, high-dimensional data for actionable insight. Our platform technology, named, bfLEAP™ is an analytical AI/ML platform developed at The Johns Hopkins University Applied Physics Laboratory (JHU-APL) which is able to surmount the challenges of scalability and flexibility currently hindering researchers and clinicians by providing a more precise, multi-dimensional understanding of their data. We are deploying bfLEAP™ for use at several critical stages of development for internal programs and through strategic partnerships and collaborations with the intention of streamlining data analytics in therapeutics development, decreasing the overall development costs by decreasing failure rates for new therapeutics, and impacting the lives of countless patients that may otherwise not receive the therapies they need.
The bfLEAP™ platform utilizes both supervised and unsupervised machine learning - as such, it is able to reveal real/meaningful connections in the data without the need for a priori hypothesis. Algorithms used in the bfLEAP™ platform are designed to handle highly imbalanced data sets to successfully identify combinations of factors that are associated with outcomes of interest.
Our primary goal is to improve the odds of success at any stage of pre-clinical and clinical therapeutics development, for in-house programs, and our strategic partners and collaborators. Our primary business model is enabling the success of ongoing clinical trials or rescue of late stage failed drugs (i.e., Phase 2 or Phase 3 clinical trial failures) for development and divestiture; although, we will also consider collaborations for earlier stage drugs. We hope to accomplish this through strategic acquisitions of current clinical stage and failed drugs for in-house development, or through strategic partnerships with biopharmaceutical industry companies. We are able to pursue our drug asset enhancement business by leveraging a powerful and proven AI/ML platform (trade name: bfLEAP™) initially developed at JHU-APL. We believe the bfLEAP™ analytics platform is a potentially disruptive tool for analysis of pre-clinical and/or clinical data sets, such as the robust pre-clinical and clinical trial data sets being generated in translational R&D and clinical trial settings.
Liquidity and Going Concern
The Company has had negative cash flows from operations and operated at a net loss since inception. In the first quarter of 2023, we completed our initial public offering (“IPO”). We believe that the funds raised and notes that were converted from debt to equity in connection with the IPO now provide enough liquidity to fund operations beyond 9 months from the date of this filing.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying condensed consolidated financial statements include the accounts of Bullfrog AI Holdings, Inc. and our wholly owned subsidiaries and have been prepared in conformity with United States generally accepted accounting principles (“GAAP”) for interim financial information. All intercompany accounts and transactions have been eliminated in consolidation.
| F-25 |
The condensed consolidated statements are unaudited and should be read in conjunction with the consolidated financial statements and related notes included in our 2022 Annual Report on Form 10-K filed with the Securities and Exchange Commission on April 25, 2023. The unaudited condensed consolidated financial statements have been prepared on a basis consistent with the audited annual consolidated financial statements included in the 10-K and, in the opinion of management, include all adjustments of a normal recurring nature necessary to fairly state our financial position, our results of operations, and cash flows.
The results for the nine months ended September 30, 2023 are not necessarily indicative of the operating results expected for the year ending December 31, 2023 or any other future period.
On February 13, 2023, we completed a 1-for-7 reverse split of our common stock. Stockholders’ equity and all references to shares and per share amounts in the accompanying unaudited condensed consolidated financial statements have been adjusted to reflect the reverse stock split for all periods presented.
Revenue Recognition
The Company recognizes revenue based on the following five step model:
| - | Identification of the contract with a customer |
| This step outlines the criteria that must be met when establishing a contract with a customer to supply goods or services. | |
| - | Identification of the performance obligations in the contract |
| This step describes how distinct performance obligations in the contract must be handled. | |
| - | Determination of the transaction price |
| This step outlines what must be considered when establishing the transaction price, which is the amount the business expects to receive for transferring the goods and services to the customer. | |
| - | Allocation of the transaction price to the performance obligations in the contract |
| This step outlines guidelines for allocating the transaction price across the contract’s separate performance obligations, and is what the customer agrees to pay for the goods and services. | |
| - | Recognition of revenue when, or as, the Company satisfies a performance obligation |
| Revenue can be recognized as the business meets each performance obligation. This step specifies how that should happen. |
Contract Services
The Company anticipates that the majority of revenues to be recognized in the near future will result from our fee for service partnership offering, designed for biopharmaceutical companies, as well as other organizations, of all sizes that have challenges analyzing data throughout the drug development process. The Company provides the customer with an analysis of large complex data sets using the Company’s proprietary Artificial Intelligence / Machine Learning platform called bfLEAP™. This platform is designed to predict targets of interest, patterns, relationships, and anomalies. The Company believes that there will be additional on-going work requested from partners therefore the service model utilizes a master services agreement with work or task orders issued for discrete analysis performed at the discovery, preclinical, or clinical stages of drug development. The Company receives a cash fee and in some instances the potential for rights to new intellectual property generated from the analysis. Once data analysis and the analysis report is complete, the Company delivers the analysis set to the customer and recognizes revenue at that point in time.
Significant Accounting Policies
There have been no new or material changes to the significant accounting policies discussed in the Company’s audited financial statements and the notes thereto included in the Annual Report on Form 10-K for the fiscal year ended December 31, 2022.
Impact of Recently Issued Accounting Standards
The Company has evaluated issued Accounting Standards Updates (“ASUs”) not yet adopted and believes the adoption of these standards will not have a material impact on its consolidated financial statements.
| F-26 |
3. Notes Payable Related Party
At various times in 2021, the Company entered into unsecured short term loan agreements with a related party for an aggregate principal balance of $49,000, each with a one-year maturity date, accruing interest at 5% and imputing an additional 1% interest. The full amount of the loans and interest was repaid in 2022.
4. Convertible Notes
Convertible Notes Payable
March 2020 Note
On March 27, 2020, the Company entered into a convertible loan agreement with the Maryland Technology Development Corporation with a principal balance of $200,000 at 6% interest. The maturity date of the loan was September 27, 2021. During the year ended December 31, 2022, the full amount of the loan and interest totaling $226,138 was converted into 205,984 shares of common stock of the Company, in accordance with the conversion notice submitted by the noteholder. Pursuant to the note agreement, the number of shares that the note converted into was based on the note balance plus accrued interest, divided by $5,000,000, times the fully diluted equity of the company, excluding convertible securities issued for capital raising purposes. There was no gain or loss due to conversion being within the terms of the agreement.
August 2021 Note
In August 2021, the Company entered into a convertible loan agreement with an unrelated party for a commitment of up to $195,000 with a 5% original issue discount and a 9% interest rate. The loan provided for a maturity date of February 9, 2022. We borrowed $72,000 and $123,000 of principal in the years ended December 31, 2021 and 2022, respectively. The noteholder had the right to convert the principal and interest into common shares of the Company at the IPO at a 20% discount to the IPO price.
As December 31, 2022, the loan was outstanding with a principal balance of $195,000 and accrued interest of $35,078. The loan was paid in its entirety in February 2023.
In connection with the convertible loan agreement, the Company also issued 195,000 Warrants with an exercise price of $1.00 exercisable for five years from issuance. In May 2022, the Company and the note holder agreed to cancel and void the warrants and enter into a new agreement for 225,000 warrants with an exercise price of $2.50. The Company assessed the differences in fair value and determined that they were de minimis and expensed the full value of the new warrants.
December 2021 Note
On December 20, 2021, the Company entered into a loan agreement with an unrelated party. The loan provided for a December 19, 2022 maturity, a 10% original issue discount and a 6% interest rate. The Company received $25,000 of proceeds from this note.
The note was automatically convertible into shares of common stock at a discount to the IPO price or based on the valuation of the Company, whichever was more favorable to the holder.
Initially, the loan was estimated to be issued with 355,114 warrants. Subsequent to the closing of the loan agreement, the Company enhanced the terms of the Bridge Note Offering under which the loan was closed and in April 2022 closed on the sale of approximately $1 million in face value of convertible bridge notes. Pursuant to the enhanced terms, the warrants were issued concurrently with the conversion of the note.
Concurrent with the closing of the Company’s IPO, the note converted according to its terms into 6,939 shares of common stock. No gain or loss was recognized on the conversion.
Convertible Bridge Notes
On April 11, 2022, the Company entered into an Exclusive placement agent and/or underwriter agreement with WallachBeth Capital LLC in connection with a proposed private and/or public offerings by the Company. On April 28, 2022, the Company received approximately $775,000 of proceeds, net of approximately $91,000 of fees and a 10% original issue discount from the sale of Convertible Bridge Notes and Warrants to several institutional investors and several individual accredited investors. In addition, the Company also received $100,000 from the sale of a Convertible Bridge Note and Warrants to a related party earlier in April. In September 2022, the Company received an additional $25,000 of proceeds, net of a 10% original issue discount from the sale of an additional Convertible Bridge Note and Warrant to an unrelated party.
| F-27 |
The Convertible Bridge Notes were initially convertible at the IPO at a 20% discount to the IPO price. The Convertible Bridge Notes provided for an original maturity date of October 31, 2022.
In connection with the Convertible Bridge Notes, the purchasers were also entitled to conditional warrants to be issued upon completion of the Company’s IPO. The agreement provided for the warrants to be exercisable for a period of five years from issuance at an exercise price equal to 110% of the IPO price or, if the Company failed to complete the IPO before October 22, 2022, 90% of the IPO price.
In the fourth quarter of 2022, the Company amended the Convertible Bridge Notes to (a) extend the maturity date until December 31, 2022, (b) provide that the conversion right would include interest through November 30, 2022, with interest accruing beyond that date being paid in cash and (c) revise the conversion price to be $4.27 based on a $25 million Company valuation. Additionally, the exercise price of the warrants was revised to $4.27.
Concurrent with the closing of the Company’s IPO in February 2023, all of the Convertible Bridge Notes converted according to their terms into 269,513 shares of common stock. No gain or loss was recognized on the conversion.
5. Convertible Notes - Related Party
Convertible Notes Payable Related Party
SAFE Agreement
On July 8, 2021, the Company entered into a Simple Agreement for Future Equity (SAFE), with a related party, at a purchase price of $150,000. The SAFE provided for no interest and terminated after conversion upon completion of the Company’s IPO. The SAFE provided for automatic conversion into the number of shares of SAFE Preferred Stock equal to the Purchase Amount divided by the Conversion Price, defined as either: (1) the SAFE Price (the price per share equal to the Post-Money Valuation Cap divided by the Company Capitalization) or (2) the Discount Price (the price per share of the Standard Preferred Stock sold in the Equity Financing multiplied by the Discount Rate), whichever calculation results in a greater number of shares of SAFE Preferred Stock.
In February 2023, the SAFE terminated and converted into 32,967 shares of common stock according to its terms upon the Company’s closing of its IPO. The conversion was considered a redemption for accounting purposes and consequently, the Company recognized a $63,626 loss on the conversion.
As of December 31, 2022, the $150,000 received from the SAFE was recorded at 6% imputed interest.
August 2021 Note
On August 19, 2021, the Company entered into a convertible loan agreement with a related party, with a principal balance of $99,900, an original issuance discount of 5% and a 9% interest rate. The loan provided for a maturity date of February 19, 2022. The noteholder had the right to convert the principal and interest into common shares of the Company at a conversion price based on a discount to the IPO price.
In February 2023, the related party elected to convert the convertible loan into 21,747 shares of common stock according to its terms upon the Company’s closing of its IPO. The conversion was considered a redemption for accounting purposes and consequently, the Company recognized a $29,333 loss on the conversion.
In connection with the convertible loan agreement, the Company also issued 99,000 Warrants with an exercise price of $1.00 exercisable for five years from issuance. In May 2022, the Company and the note holder agreed to cancel and void previous warrants and enter into a new agreement for 115,185 warrants with an exercise price of $2.50. The Company assessed the differences in fair value and determined that they were de minimis and expensed the full value of the new warrants.
| F-28 |
6. Notes Payable
In January 2023 the Company entered into a short-term note payable with a principal balance of $100,000, an original discount of 20% and a 9% interest rate. The note was paid in its entirety in February 2023.
In February 2023, the Company entered into an agreement to finance a portion of the premium for its Directors and Officers Insurance. The agreement provides for financing of $697,534 of the premium, repayments in 10 equal monthly installments of $71,485 each through December 2023 and accrued interest at 6.5%. The balance outstanding at September 30, 2023 was $213,290. The related balance of the premium of $307,735 is included in prepaid expenses.
7. Related Party
During the nine months ended September 30, 2023, the Company issued 75,000 stock options to its Chief Financial Officer for services rendered.
During the year ended December 31, 2021, the Company issued 29,286 common stock options to related parties for services rendered. The options have an original life of 10 years and vest over different periods for up to 24 months. During the three months ended September 30, 2023 and 2022, the Company recognized $430 and $450, respectively, of stock-based compensation related to these options. During the nine months ended September 30, 2023 and 2022, the Company recognized $1,290 and $1,350, respectively, of stock-based compensation related to these options.
8. Stockholder’s Equity
Preferred Stock
The Company has 10,000,000 shares of preferred stock authorized at a par value of $0.00001 with 5,500,000 being designated as Series A Convertible Preferred Stock. On October 5, 2022, the Company entered into an exchange agreement with an Investor providing for the exchange of 734,492 shares of commons stock into 73,449 shares of Series A Convertible Preferred Stock. Each share of Series A Convertible Preferred Stock is convertible at any time into 10 shares of the Company’s common stock. The Series A Preferred Stock is the economic equivalent of the common stock but has no voting rights and is subject to a blocker which prohibits the conversion into common stock if it would result in the Investor owning more than 4.99% of the Company’s outstanding common stock at such time. The Company evaluated the terms of the exchange and determined there was no significant change in fair value and therefore the Series A Preferred Stock was valued at $315,000 which is the Investor’s basis in the common stock that was exchanged.
Common Stock
The Company has 100,000,000 shares of common stock authorized at a par value of $0.00001. During the year ended December 31, 2022, the Company:
| ● | Exchanged 734,429 shares of common stock for shares of Series A Convertible Preferred stock as noted above, | |
| ● | Issued 205,984 shares of common stock pursuant to a conversion of $226,138 worth of convertible notes principal and interest, | |
| ● | Cancelled 112,225 shares of common stock as the change in number of shares issued as part of the cancellation of the prior agreements and new agreements with advisors, and | |
| ● | Issued 38,879 shares of common stock pursuant to a license agreement valued at $189,828. |
After the Company signed two licenses for two drug programs from universities in the first half of 2022 it engaged an independent valuation firm to perform an Enterprise-Equity valuation. The results of this engagement resulted in an increase in the value per share of common stock used in the Black Scholes option pricing model employed to value the Company’s equity grants and warrant issuances.
| F-29 |
In February 2023, the Company completed its IPO for the sale of 1,297,318 units (each, a “Unit,” collectively, the “Units”) at a price of $6.50 per Unit for a total of approximately $8.4 million of gross proceeds. Each Unit consisted of one share of the Company’s common stock, one tradeable warrant (each, a “Tradeable Warrant,” collectively, the “Tradeable Warrants”) to purchase one share of common stock at an exercise price of $7.80 per share, and one non-tradeable warrant (each, a “Non-tradeable Warrant,” collectively, the “Non-tradeable Warrants”; together with the Tradeable Warrants, each, a “Warrant,” collectively, the “Warrants”) to purchase one share of the Company’s common stock at an exercise price of $8.125.
In connection with the completion of its IPO, the Company issued an aggregate of 331,166 shares of common stock upon the conversion of certain outstanding convertible debt.
In connection with the IPO, in February 2023, the Company completed a 1-for-7 reverse split of our common stock. Stockholders’ equity and all references to shares and per share amounts in the accompanying unaudited condensed consolidated financial statements have been retroactively adjusted to reflect the reverse stock split for all periods presented.
In February 2023, the Company issued 7,692 shares of common stock for consulting services and recognized $50,000 of compensation expense related to these shares.
In the second quarter of 2023, we issued 436,533 shares of common stock following the exercise of 436,533 warrants for proceeds of $1,494,658.
Dilutive securities are excluded from the diluted earnings per share calculation because their effect is anti-dilutive. As of September 30, 2023, 3,796,164 warrants and 507,717 options for common shares were excluded in the calculation of net loss per share. As of September 30, 2022, 5,270,617 warrants and 484,525 options for common shares were excluded in the calculation of net loss per share.
2022 Equity Incentive Plan
In November 2022, the Company’s Board of Directors adopted, and its shareholders approved the 2022 Equity Incentive Plan (the “Plan”). The Plan provides for the granting of equity-based awards to employees, directors, and consultants. The Plan provides for equity-based awards including incentive stock options, non-qualified stock options, stock appreciation rights, performance share awards, cash awards and other equity-based awards. Awards are limited to a maximum term of 10 years and any exercise prices shall not be less than 100% of the fair market value of one share of common stock on the grant date. The Plan authorizes an initial maximum number of shares underlying awards of 900,000 with an automatic annual 15% increase beginning in 2024. As of September 30, 2023, there were 461,500 awards authorized but unissued available under the Plan.
Stock Options
The following table summarizes the stock option activity for the nine months ended September 30, 2023:
Schedule of Stock Options Activity
| Number of Shares | Weighted-Average Exercise Price | Weighted-Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value | |||||||||||||
| Outstanding at December 31, 2022 | 69,217 | $ | 3.06 | 7.08 | $ | 117,669 | ||||||||||
| Granted | 438,500 | $ | 4.41 | - | $ | - | ||||||||||
| Exercised | - | $ | - | - | $ | - | ||||||||||
| Forfeited / canceled | - | $ | - | - | $ | - | ||||||||||
| Outstanding at September 30, 2023 | 507,717 | $ | 4.23 | 9.17 | $ | 102,441 | ||||||||||
| Vested at September 30, 2023 | 215,623 | $ | 3.97 | 8.64 | $ | 52,644 | ||||||||||
| F-30 |
The fair value of options granted in the nine months ended September 30, 2023 were estimated using the Black-Scholes option pricing model based on the assumptions in the table below:
Schedule of Black Scholes Option Pricing Model
| 2023 | ||||
| Expected dividend yield | 0% | |||
| Expected volatility | 87% - 92% | |||
| Risk-free interest rate | 3.4%-4.1% | |||
| Expected life (in years) | 5.0-6.0 | |||
The weighted-average grant-date fair value of options granted during the nine months ended September 30, 2023 was $3.20. No options were issued in the nine months ended September 30, 2022.
No options were exercised in any of the periods presented.
During the three and nine months ended September 30, 2023, the Company recognized $116,410 and $475,465, respectively of compensation expense related to stock options. During the three and nine months ended September 30, 2022, the Company recognized $51,536 and $290,876 of compensation expense, respectively related to stock options.
As of September 30, 2023, the total unrecognized compensation expense related to unvested stock options, was approximately $936,000, which the Company expects to recognize over a weighted-average period of approximately 2.1 years.
Warrants
During the nine months ended September 30, 2023 and 2022, the Company granted a total of 3,195,906 and 56,623 warrants, respectively. The warrants have an original life of ten years and vest immediately and over 12 months. During the nine months ended September 30, 2023, warrants to purchase 22,939 shares vested and had a fair value of $35,712. During the year ended December 31, 2022, 174,105 shares of warrants were vested and amended with a fair value of $337,269, 51,941 shares of warrants were reclassified with a fair value of $11,097, and 42,057 shares of warrants with a fair value of $1,883 were forfeited.
During the year ended December 31, 2021, the Company granted a total of 431,659 warrants. Of this amount, 200,000 warrants, with a fair value of $12,462, were granted to advisors related to the Company’s IPO objective. The warrants have an original life of five years and vest 30 days before the intended IPO. During the year ended December 31, 2021, 0 shares of these warrants were vested. As of June 30, 2022, the warrants for 200,000 shares were cancelled and voided per agreement of the warrant holder and the Company. There was no gain or loss due to cancellation. In 2021, 138,929 warrants, with a fair value of $28,683, were issued for services rendered. The warrants have an original life of ten years and vest at different rates over as much as 36 months.
During the year ended December 31, 2021, the Company issued 92,859 warrants with a fair value of $12,980, in connection with convertible bridge debt agreements with multiple parties including a related party. The warrants had an original life of five years. During the period ending June 30, 2022, the Company determined that 50,735 warrants, with a fair value of $11,097, should not have been issued. The fair value was reclassified to Additional Paid in Capital. In May 2022, the Company and the note holders agreed to cancel and void the previous 99,000 warrants and entered into a new agreement for 115,185 warrants and the exercise price increased to $2.50 from $1, with a fair value of $15,412. In May 2022, the Company and the note holders agreed to cancel and void the previous 195,000 warrants and entered into a new agreement for 225,000 warrants with an exercise price of $2.50, with a fair value of $64,978.
| F-31 |
The 92,859 warrants discussed above were initially discounted against the notes, subsequent to year end December 31, 2021, they were deemed voided and these individuals were issued new warrants in accordance with the new terms as stated above. We assessed the differences in fair values and determined the values were de minimis and expensed the full value of the new warrants.
During the nine months ended September 30, 2023, the Company issued the following warrants:
| ● | In February 2023, in connection with the completion of the initial public offering, the Company issued 276,452 contingent warrants to certain debt holders with an exercise price of $4.27 and an expiration date 5 years from issuance. | |
| ● | In February 2023, in connection with the completion of the initial public offering, the Company issued 18,000 contingent warrants as fees to the Company’s underwriters with an exercise price of $8.125 and an expiration date 4 years from issuance. | |
| ● | As part of the sale of units in the Company’s initial public offering the Company issued 1,297,318 tradable warrants with an exercise price of $7.80 and an expiration date 5 years from issuance. Also, as part of the sale of units in the Company’s initial public offering, the Company issued 1,297,318 non-tradable warrants with an exercise price of $8.125 and an expiration date 5 years from issuance. | |
| ● | In February 2023, as part of the Company’s initial public offering, the Company issued 153,409 tradeable warrants to our underwriters pursuant to the overallotment options with an exercise price of $7.80 and an expiration date 5 years from issuance. Also in February 2023, as part of the Company’s initial public offering the Company issued 153,409 non-tradeable warrants to our underwriters pursuant to the overallotment options with an exercise price of $8.125 and an expiration date 5 years from issuance. |
During the three and nine months ended September 30, 2023, the Company recognized $5,050 and $35,712, respectively of compensation expense related to certain warrants. During the three and nine months ended September 30, 2022, the Company recognized $51,086 and $289,317 of compensation expense related to certain warrants. As of September 30, 2023, the total unrecognized compensation expense related to unvested warrants was $6,556, which the Company expects to recognize over a weighted-average period of approximately 0.4 years.
The following table provides details over the Company’s outstanding warrants as of September 30, 2023:
Schedule of Outstanding Warrants
| Exercise Price | Expiration | Number of Warrants | ||||||
| $ | 0.0007 | 2030 | 274,286 | |||||
| $ | 2.10 - $2.66 | 2026 - 2032 | 460,445 | |||||
| $ | 3.36 - $4.27 | 2028 - 2029 | 115,277 | |||||
| $ | 6.51 - $7.80 | 2026 - 2032 | 1,484,929 | |||||
| $ | 8.125 | 2027 - 2028 | 1,461,227 | |||||
| 3,796,164 | ||||||||
9. Income Taxes
The Company has not recorded any tax provision or benefit for the three and nine months ended September 30, 2023 and 2022. The Company has provided a valuation allowance for the full amount of its net deferred tax assets since realization of any future benefits from deductible temporary differences, net operating loss carryforwards and research and development credits are not more-likely-than-not to be realized at September 30, 2023 and December 31, 2022.
| F-32 |
10. Material Agreements
JHU-APL Technology License
On February 7, 2018, the Company entered into an exclusive, world-wide, royalty-bearing license from JHU-APL for the technology. The license covers three (3) issued patents, one (1) new provisional patent application, non-patent rights to proprietary libraries of algorithms and other trade secrets, the license also includes modifications and improvements. In October of 2021, the Company executed an amendment to the original license which represents improvements and new advanced analytics capabilities. In consideration of the rights granted to the Company under the License Agreement JHU received a warrant equal to five percent (5%) of the then fully diluted equity base of the Company, which shall be diluted following the closing of the IPO. Under the terms of the License Agreement, JHU will be entitled to eight percent (8%) royalty on net sales for the services provided by the Company in which the JHU licensed technology was utilized, as well as fifty percent (50%) of all sublicense revenues received by the Company. In addition, the Company is required to pay JHU an annual maintenance fee of $1,500. Minimum annual royalty payments are $20,000 for 2022, $80,000 for 2023, and $300,000 for 2024 and beyond, if cumulative annual royalty payments do not reach these levels, the amount due to JHU to reach the annual minimum is due by January 31st of the following year. Failure to make annual royalty payments is considered a material breach under the agreement and upon notice from JHU of a material breach, the Company shall have 60 days to cure the material breach. On July 8, 2022, the company entered into an exclusive, world-wide, royalty-bearing license from JHU-APL for the additional technology developed to enhance the bfLEAP™ platform. The new license provides additional intellectual property rights including patents, copyrights, and knowhow to be utilized under the Company’s bfLEAP™ analytical AI/ML platform. This license supersedes the previous license. In consideration of the new license, the Company issued 279,159 shares of common stock. Under the terms of the new License Agreement, JHU will be entitled to eight percent (8%) of net sales for the services provided by the Company to other parties and three percent (3%) for internally development drug projects in which the JHU license was utilized. The new license also contains tiered sub licensing fees that start at 50% and reduce to 25% based on revenues. In addition, under the new license agreement, the minimum annual royalty payments are $30,000 for 2022, $60,000 for 2023, and $300,000 for 2024 and beyond.
On May 31, 2023, the Company and JHU-APL entered into Amendment number 1 of the July 8, 2022 License Agreement whereby the Company gained access to certain improvements including additional patents and knowhow in exchange for a series of payments totaling $275,000. The first of these payments for $75,000 was due in July 2023 followed by payments of $75,000, $75,000 and $50,000 in years 2025, 2026 and 2027, respectively. The amendment also reduced the 2023 minimum annual royalty payment to $60,000, all other financial terms remain the same. As of September 30, 2023, we have accrued $45,000 of the 2023 minimum annual royalty payments.
George Washington University - Beta2-spectrin siRNA License
On January 14, 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from George Washington University (GWU) for rights to use siRNA targeting Beta2-spectrin in the treatment of human diseases, including hepatocellular carcinoma (HCC). The license covers methods claimed in three US and worldwide patent applications, and also includes use of this approach for treatment of obesity, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis.
In consideration of the rights granted to the Company under the License Agreement the Company paid GWU a $20,000 License Initiation Fee. Under the terms of the License Agreement, GWU will be entitled to a three percent (3%) royalty on net sales subject to quarterly minimums once the first sale has occurred subsequent to regulatory approval, as well sublicense or assignment fees in the event the Company sublicenses or assigns their rights to use the technology. The Company will also reimburse GWU for previously incurred and ongoing patent costs. The Sublicense and Assignment fee amounts decline as the Company advances the clinical development of the licensed technology. The license agreement also contains milestone payments for clinical development through the approval of an NDA and commercialization. As of September 30, 2023 and December 31, 2022, there has been no accrual for royalties since we have not begun to generate applicable revenue. The Company assessed whether the license should be capitalized and determined that the licensed program is in the early stage and therefore may not be recoverable; the Company expensed the license fee and will expense development costs until commercial viability is likely.
| F-33 |
Johns Hopkins University - Mebendazole License
On February 22, 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from Johns Hopkins University (JHU) for the use of an improved formulation of Mebendazole for the treatment of any human cancer or neoplastic disease. This formulation shows potent activity in animal models of different types of cancer and has been evaluated in a Phase I clinical trial in patients with high-grade glioma (NCT01729260). The trial, an open-label dose-escalation study, assessed the safety and efficacy of the improved formulation with adjuvant temozolomide in 24 patients with newly diagnosed gliomas. Investigators observed no dose-limiting toxicity in patients receiving all but the highest tested dose (200mg/kg/day). Four of the 15 patients receiving the maximum tested dose of 200mg/kg/day experienced dose-limiting toxicity, all of which were reversed by decreasing or eliminating the dose given. There were no serious adverse events attributed to Mebendazole at any dose during the trial. 41.7% of patients who received Mebendazole were alive at two years after enrollment, and 25% were alive at four years (Gallia et al., 2021).
The license covers six (6) issued patents and one (1) pending application. In consideration of the rights granted to the Company under the License Agreement, JHU will receive a staggered Upfront License Fee of $250,000. The initial payment for $50,000 was paid and the remaining balance of $200,000 was paid after the Company completed its IPO. The Company will also reimburse JHU for previously incurred and ongoing patent costs. Under the terms of the License Agreement, JHU will be entitled to three and one-half percent (3.5%) royalty on net sales by the Company. In addition, the Company is required to pay JHU minimum annual royalty payments of $5,000 for 2023, $10,000 for 2024, $20,000 for 2025, $30,000 for 2026 and $50,000 for 2027 and each year after until the first commercial sale after which the annual minimum royalty shall be $250,000. The license agreement also contains milestone payments for clinical development steps through the approval of an NDA and commercialization. As of September 30, 2023 and December 31, 2022, the balance of accrued expense related to this license agreement was $7,500 and $242,671, respectively. The Company assessed whether the license should be capitalized and determined that the licensed program is in the early stage and therefore may not be recoverable; the Company expensed the license fee and will expense development costs until commercial viability is likely.
Johns Hopkins University - Prodrug License
On October 13, 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from JHU and the Institute of Organic Chemistry and Biochemistry (IOCB) of the Czech Academy of Sciences for rights to commercialize N-substituted prodrugs of Mebendazole that demonstrate improved solubility and bioavailability. The license covers prodrug compositions and use for treating disease as claimed in multiple US and worldwide patent applications. In consideration for the rights granted to the Company under the License Agreement JHU and IOCB will receive a staggered upfront license fee of $100,000. The Company will also reimburse JHU and IOCB for previously incurred patent costs. Under the terms of the License Agreement, JHU and IOCB will be entitled to four percent (4.0%) royalty on net sales by the Company. In addition, the Company is required to pay JHU and IOCB minimum annual royalty payments of $5,000 for 2027, $10,000 for 2028, $20,000 for 2029, $30,000 for 2030 and $50,000 for 2031 and each year after until the first commercial sale after which the annual minimum royalty shall be $150,000. The license agreement also contains milestone payments for patent grants, clinical development steps through the approval of an NDA and commercialization. As of September 30, 2023 and December 31, 2022, the balance of accrued expense related to this license agreement was $0 and $133,238, respectively. The Company assessed whether the license should be capitalized and determined that the licensed program is in the early stage and therefore may not be recoverable; the Company expensed the license fee and will expense development costs until commercial viability is likely.
| F-34 |
PROSPECTUS
BULLFROG AI HOLDINGS, INC.
1,028,710 shares of Common Stock
And Warrants to Purchase 1,028,710 shares of Common Stock
Pre-Funded Warrants to Purchase up 478,429 shares of Common Stock
and Warrants to Purchase 478,429 shares of Common Stock
Underwriter Warrants to Purchase Up to 90,428 Shares of Common Stock
WALLACHBETH CAPITAL LLC
January 31, 2024
Through and including February 25, 2024 (the 25th day after the date of this prospectus), all dealers that effect transactions in these securities, whether or not they participated in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.