UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
For
the fiscal year ended:
For the transition period from [ ] to [ ]
Commission
file number
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
(Address of principal executive offices)
Registrant’s
telephone number: (
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) |
Name of each exchange on which registered | ||
| The Stock Market LLC (The Nasdaq Capital Market) | ||||
| The
|
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate
by check mark if registrant is a well-known seasoned issuer, as defined under Rule 405 of the Securities Act. Yes ☐
Indicate
by check mark if registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate
by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange
Act during the preceding 12 months (or for such shorter period that the issuer was required to file such reports), and (2) has been subject
to such filing requirements for the past 90 days.
Indicate
by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant
was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| ☒ | Smaller reporting company | ||
| Emerging growth company |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate
by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness
of its internal control over financial reporting under section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered
public accounting firm that prepared or issued its audit report.
If
securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant
included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate
by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No
As
of June 30, 2023, the aggregate market value of the common stock of the registrant held by non-affiliates was approximately $
The number of shares of the Registrant’s common stock outstanding as of March 27, 2024 was .
Documents
incorporated by reference:
TABLE OF CONTENTS
In this report, unless the context indicates otherwise, the terms “Company,” “we,” “us,” “our” and similar words refer to Bullfrog AI Holdings, Inc. (“Bullfrog”), a Nevada corporation.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, or the “Securities Act,” and Section 21E of the Securities Exchange Act of 1934 or the “Exchange Act.” These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from historical results or anticipated results.
In some cases, you can identify forward-looking statements by terms such as “may,” “intend,” “might,” “will,” “should,” “could,” “would,” “expect,” “believe,” “anticipate,” “estimate,” “predict,” “potential,” or the negative of these terms. These terms and similar expressions are intended to identify forward-looking statements. The forward-looking statements in this report are based upon management’s current expectations and beliefs, which management believes are reasonable. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor or combination of factors, or factors we are aware of, may cause actual results to differ materially from those contained in any forward-looking statements. You are cautioned not to place undue reliance on any forward-looking statements. These statements represent our estimates and assumptions only as of the date of this report. Except to the extent required by federal securities laws, we undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
You should be aware that our actual results could differ materially from those contained in the forward-looking statements due to a number of factors, including:
● our future financial performance, including our revenue, costs of revenue, operating expenses and profitability;
● the sufficiency of our cash and cash equivalents to meet our liquidity needs;
● our predictions about the proprietary development, digital transformation technology and bio health businesses and their respective market trends;
● our ability to attract and retain customers in all our business segments to purchase our products and services;
● the availability of financing for smaller publicly traded companies like us;
● our ability to successfully expand in our three principal business markets and into new markets and industry verticals; and
● our ability to effectively manage our growth and future expenses.
Other risks and uncertainties include such factors, among others, as market acceptance and market demand for our products and services, pricing, the changing regulatory environment, the effect of our accounting policies, industry trends, adequacy of our financial resources to execute our business plan, our ability to attract, retain and motivate key personnel, and other risks described from time to time in periodic and current reports we file with the United States Securities and Exchange Commission, or the “SEC.” You should consider carefully the statements under this report, which address additional factors that could cause our actual results to differ from those set forth in the forward-looking statements and could materially and adversely affect our business, operating results and financial condition. All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by the applicable cautionary statements.
| 1 |
PART I
ITEM 1. BUSINESS
Our Corporate History and Background
Bullfrog AI Holdings, Inc. was incorporated in the State of Nevada on February 6, 2020. Bullfrog AI Holdings, Inc. is the parent company of Bullfrog AI, Inc. and Bullfrog AI Management, LLC. which were incorporated in Delaware and Maryland, in 2017 and 2021, respectively. All of our operations are currently conducted through BullFrog AI Holdings, Inc. The Company’s principal business address is 325 Ellington Blvd, Unit 317, Gaithersburg, MD 20878. Our website address is www.bullfrogai.com. The references to our website in this Form 10-K are inactive textual references only. The information on our website is neither incorporated by reference into this Form 10-K.
Acquisition of BullFrog AI, Inc.
In March 2020, BullFrog AI, Inc. received an investment from TEDCO - the Technology Development Corporation of Maryland, a State of Maryland Investment Fund – pursuant to the issuance of a $200,000 convertible note with an 18-month term, 6% annual interest rate, and a 20% discount. In June 2020, BullFrog AI Holdings, Inc. acquired BullFrog AI, Inc. pursuant to an exchange agreement under which each share of Bull Frog AI, Inc. common stock was exchanged for a share of common stock of BullFrog AI Holdings, Inc. Immediately prior to the share exchange, each outstanding common share of BullFrog AI, Inc. was split into 25 shares of common stock. Pursuant to the agreement, 24,223,975 shares of the Company’s common stock were issued to the shareholders of BullFrog AI, Inc. in exchange for 100% of the outstanding stock of BullFrog AI, Inc. Upon completion of the exchange, BullFrog AI, Inc. became the Company’s wholly-owned subsidiary and the shareholders of BullFrog AI, Inc. held 100% of the common stock of the Company. As a result, BullFrog AI Holdings, Inc. assumed a total of $330,442 in net liabilities of BullFrog AI, Inc. Both of the entities were controlled before and after the transactions by the same controlling shareholder.
BullFrog AI Corporate History
BullFrog AI, Inc. was incorporated in the State of Delaware on August 25, 2017. Vininder Singh is the founder, CEO and chairman of BullFrog AI.
Business Overview
Most new therapeutics will fail at some point in preclinical or clinical development. This is the primary driver of the high cost of developing new therapeutics. A major part of the difficulty in developing new therapeutics is efficient integration of complex and highly dimensional data generated at each stage of development to de-risk subsequent stages of the development process. Artificial Intelligence and Machine Learning (AI/ML) has emerged as a digital solution to help address this problem.
We use artificial intelligence and machine learning to advance medicines for both internal and external projects. We are committed to increasing the probability of success and decreasing the time and cost involved in developing therapeutics. Most current AI/ML platforms still fall short in their ability to synthesize disparate, high-dimensional data for actionable insight. Our platform technology, named, bfLEAP™, is an analytical AI/ML platform derived from technology developed at The Johns Hopkins University Applied Physics Laboratory (JHU-APL), which is able to surmount the challenges of scalability and flexibility currently hindering researchers and clinicians by providing a more precise1, multi-dimensional understanding of their data. We are deploying bfLEAP™ for use at several critical stages of development for internal programs and through strategic partnerships and collaborations with the intention of streamlining data analytics in therapeutics development, decreasing the overall development costs by decreasing failure rates for new therapeutics, and impacting the lives of countless patients that may otherwise not receive the therapies they need.
1 In an August 2021 publication in DeepAI.org (https://deepai.org/publication/random-subspace-mixture-models-for-interpretable-anomaly-detection), the algorithms used in bfLEAP were compared to 10 of the most popular clustering algorithms in the world using 12 data sets. The end result showed that the algorithms used in bfLEAP had the highest average score when measuring speed and accuracy of prediction. The bfLEAP platform currently has more advanced versions of these algorithms and is applying them in multiple data analytics projects.
| 2 |
Recent Developments
On February 26, 2024, the Company announced the appointment of Dr. Thomas W. Chittenden, PhD, DPhil, PStat, as its new Chief Scientific Officer.
On January 31, 2024, the Company entered into an underwriting agreement with WallachBeth Capital, LLC as representative of the several underwriters named therein, relating to the issuance and sale of an aggregate of (i) 1,028,710 shares of common stock, par value $0.00001 per share and 478,429 pre-funded warrants in lieu of common stock (“Pre-Funded Warrants”) or 1,507,139 shares of common stock (or Pre-Funded Warrants) in lieu thereof, and accompanying warrants to purchase 1,507,139 shares of common stock at a combined public offering price of $3.782 per share (inclusive of the Pre-Funded Warrant exercise price) for gross proceeds of approximately $5,700,000, prior to deducting underwriting discounts and offering expenses.
Our Strategy
We plan to achieve our business objectives by enabling the successful development of drugs and biologics using a precision medicine approach via our proprietary artificial intelligence platform bfLEAP. The bfLEAP™ platform utilizes both supervised and unsupervised machine learning - as such, it is able to reveal real/meaningful connections in the data without the need for a prior hypothesis. Supervised machine learning uses labeled input and output data, while an unsupervised learning algorithm does not. In supervised learning, the algorithm “learns” from the training dataset by iteratively making predictions on the data and adjusting for the correct answer. Unsupervised learning, also known as unsupervised machine learning, uses machine learning algorithms to analyze and cluster unlabeled datasets. These algorithms discover hidden patterns or data groupings without the need for human intervention. Algorithms used in the bfLEAP™ platform are designed to handle highly imbalanced data sets to successfully identify combinations of factors that are associated with outcomes of interest.
Together with our strategic partners and collaborators, our primary goal is to improve the odds of success at any stage of pre-clinical and clinical therapeutics development. Our primary business model is improving the success and efficiency of drug development which is accomplished either through acquisition of drugs or partnerships and collaborations with companies that are developing drugs. We hope to accomplish this through strategic acquisitions of current clinical stage and failed drugs for in-house development, or through strategic partnerships with biopharmaceutical industry companies. We are able to pursue our drug asset enhancement business by leveraging a powerful and proven AI/ML platform (trade name: bfLEAP™) initially derived from technology developed at JHU-APL. We believe the bfLEAP™ analytics platform is a potentially disruptive tool for analysis of pre-clinical and/or clinical data sets, such as the robust pre-clinical and clinical trial data sets being generated in translational R&D and clinical trial settings. In November 2021, we amended the agreement with JHU-APL to include additional advanced AI technology. On July 8, 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from JHU-APL for the additional technology developed to enhance the bfLEAP™ platform. The July 8, 2022 JHU-APL license provides the Company with new intellectual property and also encompasses most of the intellectual property from the February 2018 license.
We believe bfLEAP™ will inform/enable decision making throughout the development cycle:
| ● | Discovery Phase - Analyze and categorize discovery phase data to better define highest-value leads from groups of candidates, for advancement to preclinical phase of development. Integrate data from high-throughput screening, pharmacodynamics assays, pharmacokinetics assays, and other key data sets to create the most accurate profile of a pool of therapeutic candidates. There is often a high degree of similarity among closely related therapeutics in a candidate pool - bfLEAP™ is able to harmonize disparate data streams for a more nuanced understanding of each candidate’s characteristics/potency. |
| ● | Pre-Clinical Data - Large-scale/multivariate analysis of pre-clinical and/or early-stage clinical data sets. In these settings, bfLEAP could be used to find novel drug targets, elucidate mechanism of action (MOA), predict potential off-target effects/side effects, uncover specific genetic/phenotypic background(s) with highest correlation to therapeutic response, etc. These insights from bfLEAP™ analysis can be used to inform decision making/study design at the subsequent step(s) of therapeutic/diagnostic development, including first-inhuman/Phase I RCTs. |
| ● | Clinical Development - Advanced/multivariate analysis of PhI and/or PhII clinical trials data, to find niche populations of highly responsive patients and/or inform patient selection for later-stage CT(s). This can be used to decrease overall study risk for larger clinical trials - including Phase II trials, and any Phase III Registration Clinical Trials. The bfLEAP™ platform analysis can also be used to more precisely understand complex correlations between therapeutic treatment and adverse events, side effects, and other undesirable responses which could jeopardize clinical trial success. |
| 3 |
Our platform is agnostic to the disease indication or treatment modality and therefore we believe that it is of value in the development of biologics or small molecules.
The process for our drug asset enhancement program is to:
| ● | acquire the rights to a drug from a biopharmaceutical industry company or academia; | |
| ● | use the proprietary bfLEAP™ AI/ML platform to determine a multi-factorial profile for a patient that would best respond to the drug; | |
| ● | rapidly conduct a clinical trial to validate the drug’s use for the defined “high-responder” population; and | |
| ● | divest/sell the rescued drug asset with the new information back to a large player in the pharma industry, following positive results of the clinical trial. |
As part of our strategy, we will continue evolving our intellectual property, analytical platform and technologies, build a large portfolio of drug candidates, and implement a model that reduces risk and increases the frequency of cash flow from rescued drugs. This strategy will include strategic partnerships, collaborations, and relationships along the entire drug development value chain, as well as acquisitions of the rights to developing failed drugs and possibly the underlying companies.
To date, we have not conducted clinical trials on any pharmaceutical drugs and our platform has not been used to identify a drug candidate that has received regulatory approval for commercialization. However, we currently have a strategic relationship with a leading rare disease non-profit organization for artificial intelligence/machine learning (“AI/ML”) analysis of late-stage clinical data. We have acquired the rights to a series of preclinical and early clinical drug assets from universities and entered into a strategic collaboration with a world-renowned research institution to create a HSV1 viral therapeutic platform to engineer immunotherapies for colorectal cancer. We have signed exclusive worldwide license agreements with Johns Hopkins University for a cancer drug that targets glioblastoma (brain cancer), pancreatic cancer, and other cancers. We have also signed an exclusive worldwide license with George Washington University for another cancer drug that targets hepatocellular carcinoma (liver cancer), and other liver diseases.
Our platform was originally developed by The Johns Hopkins University Applied Physics Laboratory (“JHU-APL”). JHU-APL uses the same technology for applications related to national defense. Over several years, the software and algorithms have been used to identify relationships, patterns, and anomalies, and make predictions that otherwise may not be found. These discoveries and insights provide an advantage when predicting a target of interest, regardless of industry or sector. We have applied the technology to various clinical data sets and have identified novel relationships that may provide new intellectual property, new drug targets, and other valuable information that may help with patient stratification for a clinical trial thereby improving the odds for success. The platform has not yet aided in the development of a drug that has reached commercialization. However, we have licensed one drug candidate that has completed a Phase 1 trial and a second candidate that is in the preclinical stages. Our aim is to use our technology on current and future available data to help us better determine the optimal path for development.
While we have not generated significant revenues from our AI/ML operations, we anticipate generating revenue in the future from the following three sources:
Contract Services
Our fee for service partnership offering model is designed for biopharmaceutical companies, as well as other organizations, of all sizes that have challenges analyzing data throughout the drug development process. We provide the customer with an analysis of large complex data sets using our proprietary Artificial Intelligence / Machine Learning platform called bfLEAP™. This platform is designed to predict targets of interest, patterns, relationships, and anomalies. Our service model involves a cash fee plus the potential for rights to new intellectual property generated from the analysis, which can be performed at the discovery, preclinical, or clinical stages of drug development.
| 4 |
Collaborative Arrangements
We plan to enter into collaborative arrangements with biotechnology and pharmaceutical companies who have drugs that are in development or have failed late Phase 2 or Phase 3 trials. The collaborations may also be at the discovery or preclinical stages of drug development. Our revenue will be a combination of fee for service cash payments and success fees based on achieving certain milestones as determined by each specific arrangement. There may also be fees or legal rights associated with the development of new intellectual property.
Acquisition of Rights to Certain Drugs
We may acquire the rights to drugs that have failed late Phase 2 or Phase 3 trials and generate revenues by using our platform to accurately determine the profile of patients that would respond to the drugs, conduct a clinical trial to test our findings either independently or with a clinical partner, and finally sell the drug back to pharmaceutical companies. We have and may continue acquiring the rights to drugs that have not yet failed any trials. We will use our technology to improve the chances for success, conduct a trial, and divest the asset. When divesting assets, the transaction may involve a combination of upfront payments, milestone payments based on clinical success, and royalties on sales of the product.
Our Products
| Product/Platform | Description | Target Market/Indications | ||
| bfLEAP™ – AI/ML platform for analysis of preclinical and/or clinical data | AI/ML analytics platform derived from technology developed at JHU-APL and licensed by the Company. | Biotechnology and pharmaceutical companies and other organizations. | ||
| siRNA | siRNA targeting Beta2-spectrin in the treatment of human diseases developed at George Washington University licensed by the Company | Hepatocellular carcinoma (HCC), treatment of obesity, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis. Has not yet initiated clinical testing. | ||
| Mebendazole | Improved formulation of Mebendazole developed at Johns Hopkins University and licensed by the Company | Glioblastoma. Has begun the process of clinical testing but has not received regulatory approval for commercialization. |
On January 14, 2022, the Company entered into an exclusive, worldwide, royalty-bearing license from George Washington University (GWU) for rights to use siRNA targeting Beta2-spectrin in the treatment of human diseases, including hepatocellular carcinoma (HCC). The license covers methods claimed in three U.S. and worldwide patent applications, and also includes use of this approach for treatment of obesity, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis. This program is currently in the preclinical stage of development. The Company recently initiated proof-of-concept studies on this asset and will use the outcome of these studies to inform a clinical development plan that would include initiation of IND-enabling studies.
Metabolic dysfunction-associated steatotic liver disease (MASLD, which until recently was called non-alcoholic fatty liver disease, or NAFLD) is a condition in which excess lipids, or fat, build up in the liver. This condition, which is more common in people who have obesity and related metabolic diseases including type 2 diabetes, affects as many as 24% of adults in the US and is associated with risk of progression to more serious conditions, including metabolic dysfunction-associated steatohepatitis (MASH), with associated liver inflammation and fibrosis, and HCC. Evidence in animal models of obesity suggest that a protein called β2-spectrin may play a key role in lipid accumulation, tissue fibrosis, and liver damage, and targeting expression or activity of this protein may be a useful approach in treating MASH and liver cancer (Rao et al., 2021).
| 5 |
In February 2022, the Company entered into an exclusive, worldwide, royalty-bearing license from Johns Hopkins University (JHU) for the use of an improved formulation of Mebendazole for the treatment of any human cancer or neoplastic disease. This formulation shows potent activity in animal models of different types of cancer and has been evaluated in a Phase I clinical trial in patients with high-grade glioma (NCT01729260). The trial, an open-label dose-escalation study, assessed the safety of the improved formulation with adjuvant temozolomide in 24 patients with newly diagnosed gliomas. Investigators observed no dose-limiting toxicity in patients receiving all but the highest tested dose (200mg/kg/day). Four of the 15 patients receiving the maximum tested dose of 200mg/kg/day experienced dose-limiting toxicity, all of which were reversed by decreasing or eliminating the dose given. There were no serious adverse events attributed to mebendazole at any dose during the trial. The Company is currently formulating a strategy to find a partner to conduct additional clinical trials with this asset to enable evaluation of safety in humans.
In October 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from Johns Hopkins University (JHU) and the Institute of Organic Chemistry and Biochemistry (IOCB) of the Czech Academy of Sciences for rights to commercialize N-substituted prodrugs of mebendazole that demonstrate improved solubility and bioavailability. The license covers prodrug compositions and use for treating disease as claimed in multiple US and worldwide patent applications. Patents have since been issued in the United States and Australia and are still in the prosecution phase in other territories. In September 2023 the Company announced results from a preclinical study demonstrating the effectiveness of BF-223, a compound chosen from this class, in an animal model for glioblastoma. The Company is currently formulating a strategy for initiating IND-enabling studies on BF-223 and is conducting outreach to identify partners that may want to license or partner in the development of BF-223.
Our bfLEAP™ Analytics Platform
We are able to pursue our drug rescue business by leveraging a powerful and proven AI/ML platform (trade name: bfLEAP™) derived from technology developed at The Johns Hopkins University Applied Physics Laboratory (JHU-APL). The bfLEAP™ platform is based on an exclusive, world-wide license granted by Johns Hopkins University Applied Physics Laboratory. The license covers three (3) issued patents, as well as a new provisional patent application, non-patent rights to proprietary libraries of algorithms and other trade secrets, which also includes modifications and improvements. On July 8, 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from JHU-APL for the additional technology developed to enhance the bfLEAP™ platform. The new license provides additional intellectual property rights including patents, copyrights and knowhow to be utilized under the Company’s bfLEAP™ analytical AI/ML platform. Under the terms of the new License Agreement, JHU will be entitled to eight (8%) percent of net sales for the services provided by the Company to other parties and 3% for internally development drug projects in which the JHU license was utilized. The new license also contains tiered sub licensing fees that start at 50% and reduce to 25% based on revenues.
We believe the bfLEAP™ analytics platform is a potentially disruptive tool for analysis of pre-clinical and/or clinical data sets, such as the robust pre-clinical and clinical trial data sets being generated in translational R&D and clinical trial settings. The input data for bfLEAP™ can include raw data (preclinical and/or clinical readouts), categorical data, sociodemographic data of patients, and various other inputs. Thus, the bfLEAP™ platform is capable of capturing the particular genetic and physical characteristics of patients in an unbiased manner, and contextualizing it against other disparate data sources from patients (e.g. molecular data, physiological data, etc.) for less biased and more meaningful conclusions. It is also uniquely scalable - the bfLEAP™ platform is able to perform analysis on large, high-volume data sets (i.e. ‘big data’) and also able to analyze highly disparate “short and wide” data as well. In terms of visualization, bfLEAP™ is able to integrate with most commonly used visualization tools for graph analytics.
We believe that the combination of a) scalable analytics (i.e., large data or short/wide data), b) state-of-the-art proprietary algorithms, c) unsupervised machine learning, and d) streamlined data ingestion/visualization makes bfLEAP™ one of the most flexible and powerful new platforms available on the market.
The Company will continue to evolve and improve bfLEAP™, and some of the proceeds from this offering may be used toward that effort either in-house or with development partners like The Johns Hopkins University Applied Physics Lab.
| 6 |
Lieber Institute for Brain Development
On September 8, 2023, the Company entered a data use and technology partnership agreement (the “Partnership Agreement”) with the Lieber Institute for Brain Development (LIBD). The Partnership Agreement covers the right of BullFrog AI to leverage its bfLEAP™ platform to mine LIBD’s comprehensive brain data, including transcriptomic, genomic, DNA methylation, cell-line, clinical, and imaging data to identify previously unrecognized relationships. The goal of the partnership is to identify previously unrecognized relationships between genes and pathways in the brain and the development of neurologic and psychiatric disorders, thereby facilitating the development of more effective treatments for diseases of the human brain. The collaboration will proceed in two stages, with the first involving unsupervised construction of graphical models to reveal relationships between brain diseases and genomic/biologic attributes, with the goal of identifying new biomarkers and drug targets across disorders. The second stage will involve creating disease-specific models that will enable identification of genes and pathways within these respective disorders. The Partnership Agreement has a one-year term of data exclusivity to complete the first stages of analyses, with a two-year extension option as performance milestones are met.,
As contemplated in the Partnership Agreement, on October 16, 2023, the Company and LIBD entered into a commercial agreement (the “Commercial Agreement”) that sets forth the key terms for commercialization of products and services developed under the Partnership Agreement. Pursuant to the Commercial Agreement, LIBD granted the Company a worldwide, royalty-bearing exclusive license so long as the Company receives net sales or income from the licensing of “Licensed Products” (as defined in the Commercial Agreement) in the application of machine learning and/or artificial intelligence for research and development in drug development, and specifically includes therapeutic products, patient selection strategies, and target identification, but excludes diagnostics and incidental uses of machine learning and/or artificial intelligence on data derived from research. Generally, “Licensed products” are any product or service which incorporates, results from, or is derived from LIBD’s Data (meaning finished brain-related data, including but not limited to DNA methylation, RNAseq, genomic, DNA methylation, cell-line, clinical, and imaging data, and the specified data set forth in the Partnership Agreement) and that the Company or its affiliate develops during the term of the Partnership Agreement, and any improvements thereof after the term of the Partnership Agreement, and all Licensed Products or services derived therefrom by the Company or its affiliates. Licensed Products may include, but are not limited to, biomarker and target identification, target validation, mapping unmet needs, identifying genetic risk factors and predictive modeling.
The Company was also granted the right to sublicense, to use the deliverables under the Partnership Agreement, and LIBD’s intellectual property rights in the data, to (i) use, sell, distribute for sale, have distributed for sale, offer for sale, have sold, import and have imported Licensed Products and (ii) to develop, have developed, make, have made Licensed Products that are derived from Licensed Products developed during the term of the Partnership Agreement, and any improvements made following the term. The Company is prohibited from sublicensing LIBD Data. The Company shall pay LIBD a royalty based on net sales of all Licensed Products sold by the Company and/or its affiliates.
The Commercial Agreement, generally, may be terminated at any time by either the Company or LIBD if either party defaults or breaches any material term of the agreement or files for protection under bankruptcy laws, makes an assignment for the benefit of creditors, appoints or suffers appointment of a receiver, trustee, or similar agent over its property.
Summary for CATIE Schizophrenia Case Study
The Company worked with the Lieber Institute for Brain Development to analyze data from the landmark CATIE trials. The CATIE trials were the largest trials ever conducted for anti-psychotic medications. BullFrog analyzed CATIE data from ~200 schizophrenia patients, with a library of almost 1 million genetic data points for each patient, more than 200 non-genetic attributes per patient, and 4 different medications used in the trial. For each of the four medications used, bfLEAP™ analysis revealed new, previously unknown relationships between individual genetic variants and negative patient symptoms. The genetic loci identified represent potential druggable targets, as well as potential stratifying criteria for future clinical trials in schizophrenia.
We performed another analysis on the data using our new advanced clustering algorithms bfLEAP 2.0 but focused on one particular drug named Olanzapine. Our bfLEAP™ 2.0 analytical results identified previously unknown, multi-dimensional associations among newly identified genetic variants, drug clearance, clinical trial sites, and clinical outcome variables in schizophrenia patients.
| 7 |

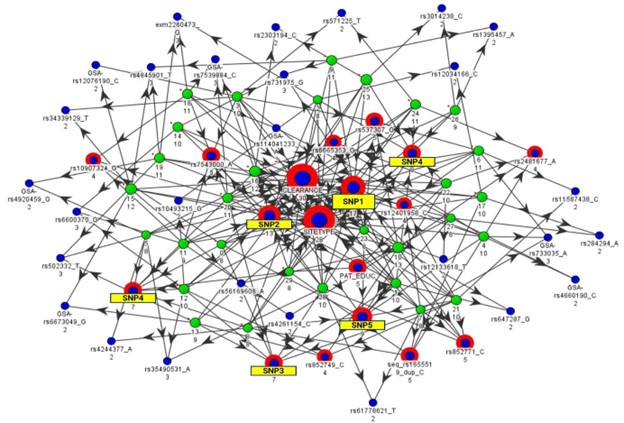
FIGURE 1 – bfLEAP™ Analytical Map
Each green node represents a different sampling of the data, and arrows point to attributes (blue nodes) which were found to be key indicators according to that sampling. Attribute importance is determined by how many samplings identify that attribute as an indicator (i.e., number of incoming arrows to each blue node).
Identification of clustered multi-variate associations (e.g., novel genetic variants, drug clearance, substance abuse) could help us 1) identify novel drug targets, 2) predict which patients are most likely to respond, and 3) identify modifiable factors that could contribute to better outcomes.
| 8 |
Summary for Cardiovascular Case Study
The Company worked with an international collaborator in cardiovascular devices to analyze data from an ongoing clinical trial for a new device. BullFrog analyzed data from ~55 patients, with a library of almost 15,000 unique attributes of data for each patient. The data also included adverse events, and key demographic information. For this collaborator, bfLEAP™ analysis was able to provide ground truth for the company - confirming multiple correlations and non-correlations within the data. In terms of actionable output, the analytical results confirmed at least two demographic co-variates for the ongoing trial, and also provided a starting point for deeper physiological and molecular studies.
Our Supply Chain and Customer Base
We have launched our businesses using funds from our initial public offering and through our partnerships and relationships. We have a strategic relationship with FSHD Society, a leading non-governmental organization, for AI/ML analysis of clinical trial data for patients with a rare neuromuscular disorder. We also have several other developing strategic relationships in the project design phase. The Company has executed a joint development deal for a biologics discovery phase opportunity that is directed toward targeted cancer therapeutics. The Company has also obtained exclusive worldwide rights to a Phase 2 ready glioblastoma drug and a discovery phase hepatocellular carcinoma drug from universities. Since we intend to conduct late-stage clinical trials with partners on rescued therapeutic assets, there will be a requirement of drug product or other significant services to plan and execute our clinical development programs. The success of our partnered clinical development programs will require adequate availability of raw materials and/or drug product for our R&D and clinical trials, and, in some cases, may also require establishment of third-party arrangements to obtain finished drug product that is manufactured appropriately under industry-standard guidelines, and packaged for clinical use or sale. Since we are a digital biopharmaceutical company, our clinical development programs will also require, in some cases, the establishment of third-party relationships for execution and completion of clinical trials.
Our Market Opportunity
One aim of our business is to “rescue” drugs that have failed in phase 3 clinical trials by using our technology to analyze all available data with the goal of designing a precision medicine clinical trial that will have a better chance of being successful. The graphic below illustrates the estimated market opportunity for these failed drugs. The top arrow shows the number of failed phase 3 trials for several disease categories over a 5-year period. The arrows below provide our assumptions for narrowing or discounting certain parameters associated with the market size calculation. The final arrow shows the math behind the $47.1B. To date, we have not penetrated the failed drug market, however; we are actively searching for failed drug opportunities.
| 9 |
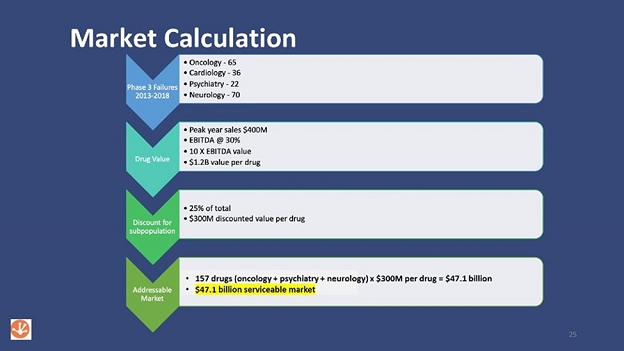
Identification of candidates with potential for rescue may be challenging and require significant resources, and once these assets are identified the Company may find it challenging to license them under favorable terms in order to create value for shareholders. Subsequent development of these assets for clinical testing may require significant effort and resources. Ultimately, these assets must undergo rigorous clinical testing and approval by FDA or comparable regulatory authorities in other countries in order to be marketed. A key part of our strategy is to partner our R&D programs. In addition, we do not intend on commercializing drugs and instead will seek to divest each drug asset to a company that will commercialize the drug. The Company may receive future royalties in come transactions.
The following graphic illustrates the global revenue forecast for applying AI in the pharmaceutical industry, as well as the increase in anticipated market spend and annual growth rate for AI solutions per certain application areas.
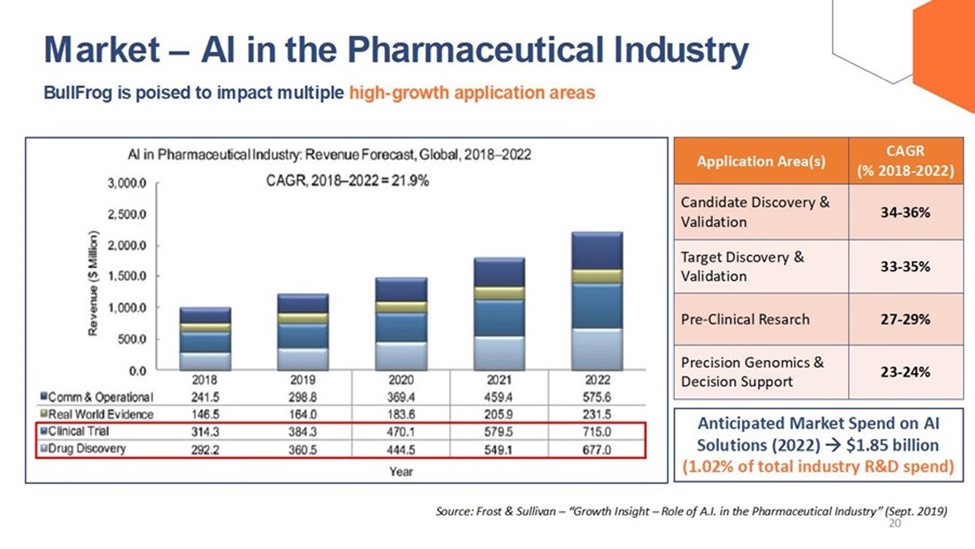
| 10 |
Intellectual Property
Patents
We have exclusive worldwide rights to the following patents related to our intellectual property:
Mebendazole Polymorph For Treatment And Prevention Of Tumors
| Serial Number | Country | Status | Issue Date | Expiration Date | ||||
| 62/112,706 | United States | Converted | N/A | N/A | ||||
| PCT/US2016/016968 | PCT | Nationalized | N/A | N/A | ||||
| 11,110,079 | United States | Granted | 9/7/2021 | 2/8/2036 | ||||
| 17/402,131 | United States | Abandoned | N/A | N/A | ||||
| 18/525,209 | United States | Pending | N/A | N/A | ||||
| 16747414.7 | Europe | Granted | 12/15/2021 | 2/8/2036 | ||||
| 16747414.7 | Czech Republic | Granted | 12/15/2021 | 2/8/2036 | ||||
| 16747414.7 | France | Granted | 12/15/2021 | 2/8/2036 | ||||
| 60 2016 067 384.3 | Germany | Granted | 12/15/2021 | 2/8/2036 | ||||
| 16747414.7 | Ireland | Granted | 12/15/2021 | 2/8/2036 | ||||
| 502022000018341 | Italy | Granted | 12/15/2021 | 2/8/2036 | ||||
| 16747414.7 | Spain | Granted | 12/15/2021 | 2/8/2036 | ||||
| 16747414.7 | Switzerland | Granted | 12/15/2021 | 2/8/2036 | ||||
| 16747414.7 | United Kingdom | Granted | 12/15/2021 | 2/8/2036 | ||||
| 253854 | Israel | Granted | 6/26/2021 | 2/8/2036 | ||||
| 2016800144274 | China | Granted | 6/25/2021 | 2/8/2036 | ||||
| 201717028684 | India | Granted | 12/1/2020 | 2/8/2036 | ||||
| 2017-541687 | Japan | Granted | 11/18/2020 | 2/8/2036 |
Mebendazole Prodrugs with Enhanced Solubility and Oral Bioavailability
| Serial Number | Country | Status | Issue Date | Expiration Date | ||||
| 62/627,810 | United States | Converted | N/A | N/A | ||||
| PCT/US2019/017291 | PCT | Nationalized | N/A | N/A | ||||
| 11,712,435 | United States | Granted | 8/1/2023 | 2/8/2039 | ||||
| 2019216757 | Australia | Granted | 1/4/2024 | 2/8/2039 | ||||
| 19751700.6 | Europe | Pending | N/A | N/A | ||||
| 3,090,691 | Canada | Pending | N/A | N/A |
Inhibition of SPTBN1 to treat Obesity/NASH and Obesity/NASH-driven cancer
| Serial Number | Country | Status | Filing Date | Expiration Date | ||||
| 63/113,745 | United States | Converted | 11/13/2020 | N/A | ||||
| 63/147,141 | United States | Converted | 2/8/2021 | N/A | ||||
| PCT/US2021/059245 | United States | Nationalized | 11/12/2021 | N/A | ||||
| 2023-528428 | Japan | Filed | 11/12/2021 | N/A | ||||
| 18/252,771 | United States | Filed | 5/12/2023 | N/A | ||||
| 21892928.9 | Europe | Filed | 6/13/2023 | N/A | ||||
| 2021800763877 | Canada | Filed | 11/12/2021 | N/A |
| 11 |
John Hopkins University Applied Physics Lab Licensed Intellectual Property:
| Title | Serial Number | File Date | Country | Status | Expiration Date | Assignee | ||||||
| Apparatus and Method for Distributed Graph Processing | U.S. Patent 10,146,801 | 7/13/2015 | US | Granted | 3/2/2037 | The Johns Hopkins University | ||||||
| Method and Apparatus for Analysis and Classification of High Dimensional Data Sets | U.S. Patent 10,936,965 | 10/5/2017 | US | Granted | 9/25/2038 | The Johns Hopkins University | ||||||
| Generalized Low Entropy Mixture Model | U.S. Patent 10,839,256 | 4/2/2018 | US | Granted | 12/15/2038 | The Johns Hopkins University |
Licenses
We hold the following licenses related to our intellectual property:
| Licensor | Licensee | Description of Rights Granted | ||
| Johns Hopkins University Applied Physics Lab | BullFrog AI, Inc. | Worldwide, exclusive rights for therapeutics development and analytical services | ||
| George Washington University | BullFrog AI Holdings | Worldwide, exclusive rights for therapeutics development | ||
| Johns Hopkins University | BullFrog AI Holdings | Worldwide, exclusive rights for therapeutics development |
JHU-APL Technology License
On February 7, 2018, the Company entered into an exclusive, world-wide, royalty-bearing license from JHU-APL for the technology. The license covers three (3) issued patents, one (1) new provisional patent application, non-patent rights to proprietary libraries of algorithms and other trade secrets, the license also includes modifications and improvements. In October of 2021, the Company executed an amendment to the original license which represents improvements and new advanced analytics capabilities. In consideration of the rights granted to the Company under the License Agreement JHU received a warrant equal to five percent (5%) of the then fully diluted equity base of the Company, which shall be diluted following the closing of the IPO. Under the terms of the License Agreement, JHU will be entitled to eight percent (8%) royalty on net sales for the services provided by the Company in which the JHU licensed technology was utilized, as well as fifty percent (50%) of all sublicense revenues received by the Company. In addition, the Company is required to pay JHU an annual maintenance fee of $1,500. Minimum annual royalty payments are $20,000 for 2022, $80,000 for 2023, and $300,000 for 2024 and beyond, if cumulative annual royalty payments do not reach these levels, the amount due to JHU to reach the annual minimum is due by January 31st of the following year. Failure to make annual royalty payments is considered a material breach under the agreement and upon notice from JHU of a material breach, the Company shall have 60 days to cure the material breach.
On July 8, 2022, the company entered into an exclusive, world-wide, royalty-bearing license from JHU-APL for the additional technology developed to enhance the bfLEAP™ platform. The new license provides additional intellectual property rights including patents, copyrights, and knowhow to be utilized under the Company’s bfLEAP™ analytical AI/ML platform. This license supersedes the previous license. In consideration of the new license, the Company issued 39,879 shares of common stock. Under the terms of the new License Agreement, JHU will be entitled to eight percent (8%) of net sales for the services provided by the Company to other parties and three percent (3%) for internally development drug projects in which the JHU license was utilized. The new license also contains tiered sub licensing fees that start at 50% and reduce to 25% based on revenues. In addition, the Company is required to pay JHU an annual maintenance fee of $1,500. Minimum annual payments are set to be $30,000 for 2022, $80,000 for 2023, and $300,000 for 2024 and beyond, all of which are creditable by royalties. The financial terms of the new license agreement replace the original terms and are not duplicative.
| 12 |
On May 31, 2023, the Company and JHU-APL entered into Amendment number 1 of the July 8, 2022 License Agreement whereby the Company gained access to certain improvements including additional patents and knowhow in exchange for a series of payments totaling $275,000. The first of these payments for $75,000 was due in July 2023 followed by payments of $75,000, $75,000, and $50,000 in years 2025, 2026 and 2027, respectively. The amendment also reduced the 2023 minimum annual royalty payment to $60,000, all other financial terms remain the same. As of December 31, 2023, we have accrued $60,000 of the 2023 minimum annual royalty payments.
George Washington University - Beta2-spectrin siRNA License
On January 14, 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from GWU for rights to use siRNA targeting Beta2-spectrin in the treatment of human diseases, including HCC. The license covers methods claimed in three US and worldwide patent applications, and also includes use of this approach for treatment of obesity, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis. This program is currently in the preclinical stage of development. The Company has not yet initiated development activities or IND-enabling studies on this asset; however, the plan is to conduct this work over the next 24 months. All R&D to date on this candidate has been conducted by the licensor of the technology, George Washington University. The term of the agreement began on January 14, 2022 and ends on the expiration date of the last patent to expire or 10 years after the first sale of a licensed product if no patents have been issued. The license can be terminated by the licensee upon 60 days’ written notice, or by the licensor if the Company is more than 30 days late in paying amounts owed to the licensor and does not make payment upon demand, or in the event of any material breach of the license that is not cured within 45 days.
Non-alcoholic fatty liver disease (NAFLD) is a condition in which excess lipids, or fat, build up in the liver. This condition, which is more common in people who have obesity and related metabolic diseases including type 2 diabetes, affects as many as 24% of adults in the US and is associated with risk of progression to more serious conditions, including non-alcoholic steatohepatitis (NASH), with associated liver inflammation and fibrosis, and hepatocellular carcinoma (HCC). Evidence in animal models of obesity suggest that a protein called β2-spectrin may play a key role in lipid accumulation, tissue fibrosis, and liver damage, and targeting expression or activity of this protein may be a useful approach in treating NASH and liver cancer (Rao et al., 2021).
In consideration of the rights granted to the Company under the license agreement, GWU received a $20,000 License Initiation Fee. Under the terms of the License Agreement, GWU will be entitled to a three percent (3%) royalty on net sales subject to quarterly minimums once the first sale has occurred subsequent to regulatory approval, as well sublicense or assignment fees in the event the Company sublicenses or assigns their rights to use the technology. The Company will also reimburse GWU for previously incurred and ongoing patent costs. The Sublicense and Assignment fee amounts decline as the Company advances the clinical development of the licensed technology. The license agreement also contains milestone payments for clinical development through the approval of a New Drug Application (NDA) and commercialization.
Aggregate payments made to GWU to date include the $20,000 License Initiation Fee and an additional $6,550 to reimburse the licensor for past patent costs. Aggregate future milestone costs could reach $860,000 if the drug successfully completes clinical trials and is the subject of an NDA to the U.S. FDA. Future milestones on sales revenue are limited to $1 million on the first $20 million in net sales.
As of December 31, 2023 and 2022, there has been no accrual for royalties since we have not begun to generate applicable revenue. The Company assessed whether the license should be capitalized and determined that the licensed program is in the early stage and therefore may not be recoverable; the Company expensed the license fee and will expense development costs until commercial viability is likely.
| 13 |
Johns Hopkins University – Mebendazole License
On February 22, 2022, the Company entered into an exclusive, worldwide, royalty-bearing license from JHU for the use of an improved formulation of Mebendazole for the treatment of any human cancer or neoplastic disease. This formulation shows potent activity in animal models of different types of cancer, and has been evaluated in a Phase I clinical trial in patients with high-grade glioma (NCT01729260). The trial, an open-label dose-escalation study, assessed the safety of the improved formulation with adjuvant temozolomide in 24 patients with newly diagnosed gliomas. Investigators observed no dose-limiting toxicity in patients receiving all but the highest tested dose (200mg/kg/day). Four of the 15 patients receiving the maximum tested dose of 200mg/kg/day experienced dose-limiting toxicity, all of which were reversed by decreasing or eliminating the dose given. There were no serious adverse events attributed to mebendazole at any dose during the trial. The Company is currently formulating a strategy to conduct additional clinical trials with this asset to enable evaluation of safety in humans.
The license covers six (6) issued patents and one (1) pending application, with the term of the agreement beginning on February 22, 2022 and ending on the date of expiration of the last to expire patent. The license can be terminated by the licensee upon 90 days’ written notice, or by the licensor in the event of any material breach of the license that is not cured within 30 days. In consideration of the rights granted to the Company under the license agreement, JHU will receive a staggered Upfront License Fee of $250,000, with the first $50,000 payment due within 30 days of the effective date. The Company will also reimburse JHU for previously incurred and ongoing patent costs. Under the terms of the license agreement, JHU will be entitled to three- and one-half percent (3.5%) royalty on net sales by the Company. In addition, the Company is required to pay JHU minimum annual royalty payments of $5,000 for 2023, $10,000 for 2024, $20,000 for 2025, $30,000 for 2026 and $50,000 for 2027 and each year after until the first commercial sale after which the annual minimum royalty shall be $250,000. The license agreement also contains milestone payments for clinical development steps through the approval of an NDA and commercialization. Aggregate payments made to date include the initial $50,000 upfront fee and an additional $79,232 to reimburse the licensor for past patent costs. Aggregate future milestone costs could reach $1,500,000 if the drug successfully completes Phase II and III clinical trials and is approved for sale and marketing by the US FDA. Future milestones on sales revenue are $1 million on the first $20 million in sales revenue, $2 million in the first-year cumulative sales revenue exceeds $100 million, $10 million in the first-year cumulative sales revenue exceeds $500 million, and $20 million in the first-year cumulative sales revenue exceeds $1 billion. As of December 31, 2023 and 2022, the balance of accrued expense related to this license agreement was $10,000 and $242,671, respectively. The Company assessed whether the license should be capitalized and determined that the licensed program is in the early stage and therefore may not be recoverable; the Company expensed the license fee and will expense development costs until commercial viability is likely.
Johns Hopkins University – Mebendazole Prodrug License
On October 13, 2022, the Company entered into an exclusive, worldwide, royalty-bearing license from JHU and the Institute of Organic Chemistry and Biochemistry (IOCB) of the Czech Academy of Sciences for rights to commercialize N-substituted prodrugs of mebendazole that demonstrate improved solubility and bioavailability. The license covers prodrug compositions and use for treating disease as claimed in multiple US and worldwide patent applications. The term of the agreement began on October 13, 2022 and continues until the date of expiration of the last to expire patent, or for 20 years from the effective date of the agreement if no patents are issued. The license can be terminated by the Company upon 90 days’ written notice, or by the licensor in the event of any material breach of the license that is not cured by the Company within 30 days.
| 14 |
In consideration for the rights granted to the Company under the License Agreement JHU and IOCB will receive a staggered upfront license fee of $100,000. The Company will also reimburse JHU and IOCB for previously incurred patent costs totaling $33,265 and will be responsible for reimbursing licensors for future patent costs. Under the terms of the License Agreement, the licensors will be entitled to a four percent (4%) royalty on net sales subject to annual minimums upon first commercial sale of a licensed product, as well sublicense or assignment fees in the event the Company sublicenses or assigns their rights to use the technology. The Sublicense fee amount declines as the Company advances the clinical development of licensed technology. The Company is required to pay minimum annual royalties (MAR) beginning in year 4 of the agreement. The MAR for year 4 will be $5,000, increasing to $10,000 in year 5, $20,000 in year 6, $30,000 in year 7, and $50,000 in year 8 and subsequent years. The Company will be responsible for milestone payments for patent issuance of up to $50,000 and clinical development milestones up to and including approval of an NDA totaling up to $2.3 million. The Company will be required to pay a commercial milestone of $1 million once sales reach $20 million in the US, $2 million when sales in the US reach $100 million, $10 million when US sales reach $500 million, and $20 million when US sales exceed $1 billion.
As of December 31, 2023 and 2022, the balance of accrued expense related to this license agreement was $0 and $133,238, respectively. The Company assessed whether the license should be capitalized and determined that the licensed program is in the early stage and therefore may not be recoverable; the Company expensed the license fee and will expense development costs until commercial viability is likely.
On September 26, 2023, the Company announced positive data in a preclinical study investigating the anti-cancer activity of a novel prodrug of mebendazole for the treatment of glioblastoma. The study assessed the relative efficacy of BF-222, a novel formulation of mebendazole that has been evaluated in clinical trials, and BF-223, a novel prodrug of mebendazole with improved solubility and bioavailability relative to BF-222, compared with placebo in mice that had been implanted with tumor cells as a model for human glioblastoma. Animals treated with BF-223 had an average survival time of 27.9 days compared with 27.3 days for mice treated with BF-222 and 23.4 days for mice given placebo. Mice treated with BF-223 were administered 80% of the dose that mice treated with BF-222 received, and improved outcomes for both treatment groups were statistically significant compared to placebo. In addition, animals treated with equivalent doses of BF-222 and BF-223 showed comparable and significant reduction in tumor growth compared to control animals during the study.
Competition
The pharmaceutical and biotechnology industries are characterized by rapidly advancing technologies, intense competition, and a strong emphasis on proprietary products. The immuno-oncology, neuroscience, and rare disease segments of the industry in particular are highly competitive. While we believe that our technology, development experience and scientific knowledge provide competitive advantages, we face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical, and biotechnology companies, academic institutions and governmental agencies, and public and private research institutions.
Many of our competitors may have significantly greater financial resources, and expertise in research and development, manufacturing, preclinical studies, conducting clinical trials, obtaining regulatory approvals, and marketing approved medicines than we do. Mergers and acquisitions in the pharmaceutical, biotechnology, and diagnostic industries may result in even more resources being concentrated among a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and in establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to or necessary for our programs. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
| 15 |
The key competitive factors affecting the success of all of our product candidates, if approved, are likely to be their efficacy, safety, convenience, price, the effectiveness of companion diagnostics in guiding the use of related therapeutics, if any, the level of generic competition and the availability of reimbursement from government and other third-party payors.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize medicines that are safer, are more effective, have fewer or less severe side effects, are more convenient or are less expensive than any medicines we may develop. Our competitors also may obtain FDA or other regulatory approval for their medicines more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, our ability to compete may be affected in many cases by insurers or other third-party payors seeking to encourage the use of generic medicines. There are many generic medicines currently on the market for certain of the indications that we are pursuing, and additional generics are expected to become available over the coming years. If our therapeutic product candidates are approved, we expect that they will be priced at a significant premium over competitive generic medicines.
Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future. If the product candidates of our priority programs are approved for the indications for which we are currently planning clinical trials, they will compete with the drugs discussed below and will likely compete with other drugs currently in development.
bfLEAP
The analytics industry and application of AI in healthcare is growing rapidly. Competition exists along the entire continuum of the drug development process from discovery to commercialization and beyond. We believe the weakness of the industry is the quality of the data and we believe bfLEAP provides several competitive advantages, that will position the Company for success, First, bfLEAP is highly scalable and can process data from small to extremely large complex data sets without the need for additional code being developed. Second, it is adept at processing and analyzing incomplete data and making predictions that we do not believe other technologies are capable of doing. Finally, bfLEAP has the ability to extract the most important features for analysis out of extremely large complex data sets using unsupervised machine learning algorithms, thereby greatly simplifying complex problems. Since data quality is a problem that exists in the healthcare industry, we see these as major differentiators. The ability to make predictions, find relationships and patterns and anomalies in extremely large complex data sets has been demonstrated by the Applied Physics Lab in other applications and sectors. Finally, the algorithms used by bfLEAP are proprietary and protected, having been developed at Johns Hopkins University Applied Physics Lab. We believe most of the competitors rely on open-source algorithms and we also believe that we have already demonstrated our superiority via the August 2021 publication in DeepAI.org.
Government Regulation
The FDA does not currently require approval of AI technologies used to aid in therapeutics, but that could change in the future. The FDA will regulate any clinical trials conducted by the Company.
Our clinical development programs will, in some cases, require regulatory review of preclinical and/or clinical data by the FDA or other governing agencies, and subsequent compliance with applicable federal, state, local, and foreign statutes and regulations. The results of the clinical trials that we conduct will be evaluated by the FDA and other regulatory bodies. The comments and approvals that are obtained are expected to lead to milestone payments under the collaborative agreement. Accordingly, our ability to navigate the regulatory process is extremely important to the success of the Company. We believe that we have a competitive advantage in this process due to primarily focusing on drug candidates that already have some level of success in clinical trials. Previous success of a particular candidate in trials combined with our precision medicine approach to clinical trial design using our bfLEAP platform, will de-risk the development process and improve the chances for success.
| 16 |
Government Regulation and Product Approval
Government authorities in the United States, at the federal, state and local level, and in other countries and jurisdictions extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, post-approval monitoring and reporting, and import and export of pharmaceutical products. The processes for obtaining regulatory approvals in the United States and in foreign countries and jurisdictions, along with subsequent compliance with applicable statutes and regulations and other regulatory authorities, require the expenditure of substantial time and financial resources.
FDA Approval Process
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The Federal Food, Drug, and Cosmetic Act (FD&C Act) and other federal and state statutes and regulations govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending new drug applications (NDAs), warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties and criminal prosecution.
Pharmaceutical product development for a new product or certain changes to an approved product in the U.S. typically involves preclinical laboratory and animal tests, the submission to FDA of an investigational new drug application (IND) which must become effective before clinical testing may commence, and adequate and well-controlled clinical trials to establish the safety and effectiveness of the drug for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease.
Preclinical tests include laboratory evaluation of product chemistry, formulation and toxicity, as well as animal trials to assess the characteristics and potential safety and efficacy of the product. The conduct of the preclinical tests must comply with federal regulations and requirements, including good laboratory practices. The results of preclinical testing are submitted to FDA as part of an IND along with other information, including information about product chemistry, manufacturing and controls, and a proposed clinical trial protocol. Long-term preclinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted. A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If FDA has neither commented on nor questioned the IND within this 30-day period, the clinical trial proposed in the IND may begin. Clinical trials involve the administration of the investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted: (i) in compliance with federal regulations; (ii) in compliance with good clinical practice, or GCP, an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators, and monitors; as well as (iii) under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to FDA as part of the IND.
| 17 |
Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, but the phases may overlap. In Phase 1, the initial introduction of the drug into healthy human subjects or patients, the drug is tested to assess metabolism, pharmacokinetics, pharmacological actions, side effects associated with increasing doses, and, if possible, early evidence of effectiveness. Phase 2 usually involves trials in a limited patient population to determine the effectiveness of the drug for a particular indication, dosage tolerance and optimum dosage, and to identify common adverse effects and safety risks. If a drug demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 trials are undertaken to obtain the additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit FDA to evaluate the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the drug. In most cases, FDA requires two adequate and well-controlled Phase 3 clinical trials to demonstrate the efficacy of the drug. A single Phase 3 trial with other confirmatory evidence may be sufficient in rare instances, such as where the study is a large multicenter trial demonstrating internal consistency and a statistically very persuasive finding of a clinically meaningful effect on mortality, irreversible morbidity, or prevention of a disease with a potentially serious outcome and confirmation of the result in a second trial would be practically or ethically impossible.
After completion of the required clinical testing, an NDA is prepared and submitted to FDA. FDA approval of the NDA is required before marketing of the product may begin in the U.S. The NDA must include the results of all preclinical, clinical and other testing and a compilation of data relating to the product’s pharmacology, chemistry, manufacture and controls. The cost of preparing and submitting an NDA is substantial. The submission of most NDAs is additionally subject to a substantial application user fee, and the applicant under an approved NDA is also subject to an annual program fee for each prescription product. These fees are typically increased annually. Sponsors of applications for drugs granted Orphan Drug Designation are exempt from these user fees.
FDA may also refer applications for novel drug products, or drug products that present difficult questions of safety or efficacy, to an outside advisory committee – typically a panel that includes clinicians and other experts – for review, evaluation, and a recommendation as to whether the application should be approved. FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations.
Before approving an NDA, FDA will typically inspect one or more clinical sites to assure compliance with GCP. Additionally, FDA will inspect the facility or the facilities at which the drug is manufactured. FDA will not approve the product unless compliance with current good manufacturing practices (cGMPs) is satisfactory and the NDA contains data that provide substantial evidence that the drug is safe and effective in the indication studied.
Fast Track Designation
FDA is required to facilitate the development, and expedite the review, of drugs that are intended for the treatment of a serious or life-threatening disease or condition for which there is no effective treatment and which demonstrate the potential to address unmet medical needs for the condition. Under the Fast Track program, the sponsor of a new drug candidate may request that FDA designate the drug candidate for a specific indication as a Fast Track drug concurrent with, or after, the filing of the IND for the drug candidate. FDA must determine if the drug candidate qualifies for Fast Track Designation within 60 days of receipt of the sponsor’s request.
If a submission is granted Fast Track Designation, the sponsor may engage in more frequent interactions with FDA, and FDA may review sections of the NDA before the application is complete. This rolling review is available if the applicant provides, and FDA approves, a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, FDA’s time period goal for reviewing an application does not begin until the last section of the NDA is submitted. While we may seek Fast Track Designation, there is no guarantee that we will be successful in obtaining any such designation. Even if we do obtain such designation, we may not experience a faster development process, review or approval compared to conventional FDA procedures. A Fast Track Designation does not ensure that the product candidate will receive marketing approval or that approval will be granted within any particular timeframe. Additionally, Fast Track Designation may be withdrawn by FDA if FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
| 18 |
Post-Approval Requirements
Once an NDA is approved, a product will be subject to certain post-approval requirements. For instance, FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet. Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved labeling.
Adverse event reporting and submission of periodic reports are required following FDA approval of an NDA. FDA also may require post-marketing testing, known as Phase 4 testing, REMS and surveillance to monitor the effects of an approved product, or FDA may place conditions on an approval that could restrict the distribution or use of the product. In addition, quality control, drug manufacture, packaging and labeling procedures must continue to conform to cGMPs after approval. Drug manufacturers and certain of their subcontractors are required to register their establishments with FDA and certain state agencies. Registration with FDA subjects entities to periodic unannounced inspections by FDA, during which the Agency inspects manufacturing facilities to assess compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money, and effort in the areas of production and quality-control to maintain compliance with cGMPs. Regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered.
Generic Competition
In seeking approval for a drug through an NDA, applicants are required to list with the FDA each patent whose claims cover the applicant’s product. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential generic competitors in support of approval of an abbreviated new drug application (ANDA). An ANDA provides for marketing of a drug product that has the same active ingredients in the same strengths and dosage form as the listed drug and has been shown through bioequivalence testing to be therapeutically equivalent to the listed drug. Other than the requirement for bioequivalence testing, ANDA applicants are not required to conduct, or submit results of, preclinical or clinical tests to prove the safety or effectiveness of their drug product. Drugs approved in this way are commonly referred to as “generic equivalents” to the listed drug and can often be substituted by pharmacists under prescriptions written for the original listed drug.
The ANDA applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA’s Orange Book. Specifically, the applicant must certify that (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product (a Paragraph IV certification). The ANDA applicant may also elect to submit a section viii statement certifying that its proposed ANDA label does not contain (or carve out) any language regarding the patented method-of-use rather than certify to a listed method-of-use patent. If the applicant does not challenge the listed patents or certifies that the listed patents will not be infringed by the new product, the ANDA application will not be approved until all the listed patents claiming the referenced product have expired. If the ANDA applicant has provided a Paragraph IV certification, the NDA and patent holders may then initiate a patent infringement lawsuit in response. The filing of a patent infringement lawsuit within 45 days of the receipt of a such certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit, or a decision in the infringement case that is favorable to the ANDA applicant.
Exclusivity
Upon NDA approval of a new chemical entity (NCE) that drug receives five years of marketing exclusivity during which FDA cannot receive any ANDA seeking approval of a generic version of that drug. An ANDA may be submitted one year before NCE exclusivity expires if a Paragraph IV certification is filed. If there is no listed patent in the Orange Book, there may not be a Paragraph IV certification, and, thus, no ANDA may be filed before the expiration of the exclusivity period. Certain changes to a drug, such as the addition of a new indication to the package insert, can be the subject of a three-year period of exclusivity if the application contains reports of new clinical investigations (other than bioavailability studies) conducted or sponsored by the sponsor that were essential to approval of the application. FDA cannot approve an ANDA for a generic drug that includes the change during the period of exclusivity.
| 19 |
Patent Term Extension
After NDA approval, owners of relevant drug patents may apply for up to a five-year patent extension. The allowable patent term extension is calculated as half of the drug’s testing phase (the time between IND application and NDA submission) and all of the review phase (the time between NDA submission and approval up to a maximum of five years). The time can be shortened if FDA determines that the applicant did not pursue approval with due diligence. The total patent term after the extension may not exceed 14 years, and only one patent can be extended. For patents that might expire during the application phase, the patent owner may request an interim patent extension. An interim patent extension increases the patent term by one year and may be renewed up to four times. For each interim patent extension granted, the post-approval patent extension is reduced by one year. The director of the United States Patent and Trademark Office must determine that approval of the drug covered by the patent for which a patent extension is being sought is likely. Interim patent extensions are not available for a drug for which an NDA has not been submitted.
Other Healthcare Laws
In the United States, biotechnology company activities are subject to regulation by various federal, state and local authorities in addition to the FDA, including but not limited to, the Centers for Medicare & Medicaid Services (CMS), other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General and the Office for Civil Rights), the U.S. Department of Justice (DOJ) and individual U.S. Attorney offices within the DOJ, and state and local governments. For example, research, sales, marketing, and scientific/educational grant programs have to comply with the anti-fraud and abuse provisions of the Social Security Act, the federal false claims laws, the privacy and security provisions of the Health Insurance Portability and Accountability Act (HIPAA) and similar state laws, each as amended, as applicable.
Also, many states have similar fraud and abuse statutes or regulations that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
Data privacy and security regulations by both the federal government and the states in which business is conducted may also be applicable. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, imposes requirements relating to the privacy, security and transmission of individually identifiable health information. HIPAA requires covered entities to limit the use and disclosure of protected health information to specifically authorized situations and requires covered entities to implement security measures to protect health information that they maintain in electronic form. Among other things, HITECH made HIPAA’s security standards directly applicable to business associates, independent contractors or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Insurance Coverage and Reimbursement
Significant uncertainty exists as to the insurance coverage and reimbursement status of any products for which we may obtain regulatory approval. In the United States, sales of any product candidates for which regulatory approval for commercial sale is obtained will depend in part on the availability of coverage and adequate reimbursement from third-party payors. Third-party payors include government authorities and health programs in the United States such as Medicare and Medicaid, managed care providers, private health insurers and other organizations. These third-party payors are increasingly reducing reimbursements for medical products and services. The process for determining whether a payor will provide coverage for a drug product may be separate from the process for setting the reimbursement rate that the payor will pay for the drug product. Third-party payors may limit coverage to specific drug products on an approved list, or formulary, which might not include all of FDA-approved drugs for a particular indication. A payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Further, coverage and reimbursement for drug products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance.
| 20 |
Human Capital Resources
As of February 10, 2023, the Company has 4 full-time employees and consultants, including its Chief Executive Officer Vininder Singh and its Chief Financial Officer, Dane Saglio and 7 part-time employees, advisors, and consultants. None of these employees are covered by a collective bargaining agreement, and we believe our relationship with our employees is good. We also engage consultants on an as-needed basis to supplement existing staff.
Properties
Currently, the Company does not own any real property. All of the Company’s employees work virtually.
Legal Proceedings
The Company is not a party to any legal proceedings.
Corporate Information
BullFrog AI Holdings, Inc. was incorporated in the State of Nevada on February 6, 2020. Our principal business address is 325 Ellington Blvd, Unit 317, Gaithersburg, MD 20878. Our website address is www.bullfrogai.com. The references to our website in this annual report are inactive textual references only. The information on our website is neither incorporated by reference into this annual report nor intended to be used in connection with this annual report. All of our operations are currently conducted through BullFrog AI Holdings, Inc.
Available Information
We file annual, quarterly, and current reports, proxy statements and other information with the U.S. Securities Exchange Commission (the “SEC”). These filings are available to the public through the SEC’s website at http://www.sec.gov. All statements made in any of our securities filings, including all forward-looking statements or information, are made as of the date of the document in which the statement is included unless otherwise specified, and we do not assume or undertake any obligation to update any of those statements or documents unless we are required to do so by law.
ITEM 1A. RISK FACTORS
Smaller reporting companies are not required to provide the information required by this item.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Smaller reporting companies are not required to provide the information required by this item.
ITEM 1C. CYBERSECURITY
We have established policies and processes for assessing, identifying, and managing material risk from cybersecurity threats, and have integrated these processes into our overall risk management systems and processes. We routinely assess material risks from cybersecurity threats, including any potential unauthorized occurrence on or conducted through our information systems that may result in adverse effects on the confidentiality, integrity, or availability of our information systems or any information residing therein.
| 21 |
We conduct periodic risk assessments to identify cybersecurity threats, as well as assessments in the event of a material change in our business practices that may affect information systems that are vulnerable to such cybersecurity threats. These risk assessments include identification of reasonably foreseeable internal and external risks, the likelihood and potential damage that could result from such risks, and the sufficiency of existing policies, procedures, systems, and safeguards in place to manage such risks.
Following these risk assessments, we re-design, implement, and maintain reasonable safeguards to minimize identified risks; reasonably address any identified gaps in existing safeguards; and regularly monitor the effectiveness of our safeguards. Primary responsibility for assessing, monitoring and managing our cybersecurity risks rests with our Chief Information Officer who reports to our Chief Commercial Officer, to manage the risk assessment and mitigation process.
We engage consultants, or other third parties in connection with our risk assessment processes. These service providers assist us to design and implement our cybersecurity policies and procedures, as well as to monitor and test our safeguards. We require each third-party service provider to certify that it has the ability to implement and maintain appropriate security measures, consistent with all applicable laws, to implement and maintain reasonable security measures in connection with their work with us, and to promptly report any suspected breach of its security measures that may affect our company.
We have not encountered cybersecurity challenges that have materially impaired our operations or financial standing.
Governance
Our board of directors addresses the Company’s cybersecurity risk management as part of its general oversight function. The board of directors’ audit committee is responsible for overseeing Company’s cybersecurity risk management processes, including oversight and mitigation of risks from cybersecurity threats.
Our cybersecurity risk assessment and management processes are implemented and maintained by certain Company management, including the information technology team at the direction of our Chief Information Officer. Our executive team including our Chief Executive Officer, and Chief Financial Officer are responsible for hiring appropriate personnel, helping to integrate cybersecurity risk considerations into the Company’s overall risk management strategy, and communicating key priorities to relevant personnel. This executive team is responsible for approving budgets, helping prepare for cybersecurity incidents, approving cybersecurity processes, and reviewing security assessments and other security-related reports.
Our cybersecurity incident response and vulnerability management policies are designed to escalate certain cybersecurity incidents to members of management depending on the circumstances, including our Chief Executive Officer, and Chief Financial Officer. In addition, the Company’s incident response and vulnerability management policies include reporting to the audit committee of the board of directors for certain cybersecurity incidents including significant breaches to the Company’s networks or systems. The audit committee receives regular reports from the information technology team concerning the Company’s significant cybersecurity threats and risk and the processes the Company has implemented to address them. The audit committee also has access to various reports, summaries or presentations related to cybersecurity threats, risk and mitigation.
ITEM 2. PROPERTIES
The Company’s principal business address is 325 Ellington Blvd, Unit 317, Gaithersburg, MD 20878, and the telephone number at such address is 240-658-6710. Currently, the Company does not own any real property. All of the Company’s employees work virtually.
ITEM 3. LEGAL PROCEEDINGS
We are not currently a party to any legal or administrative proceedings. Our current officers and directors have not been convicted in a criminal proceeding nor have they been permanently or temporarily enjoined, barred, suspended, or otherwise limited from involvement in any type of business, securities or banking activities.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
| 22 |
PART II
ITEM 5. MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Information with Respect to our Common Stock and Tradeable Warrants
Our common stock is publicly traded on the Nasdaq Capital Market, or Nasdaq, and began trading under the symbol “BFRG” on February 14, 2023. Our tradeable warrants are traded on Nasdaq and began trading under the symbol “BFRGW” on February 14, 2023.
Holders of Record
As of March 27, 2024 we had 17 shareholders of record of our common stock. This number does not include beneficial owners whose shares are held by nominees in street name.
Dividend Policy
Holders of common stock are entitled to receive ratably such dividends, if any, as may be declared by the Board of Directors out of funds legally available. We have not paid any dividends since our inception, and we presently anticipate that all earnings, if any, will be retained for the development of our business. Any future disposition of dividends will be at the discretion of our Board of Directors and will depend upon, among other things, our future earnings, operating and financial condition, capital requirements, and other factors.
Recent Sales of Unregistered Securities
None.
Securities Authorized for Issuance under Equity Compensation Plans
The information required by this item with respect to securities authorized for issuance under equity compensation plans is set forth in Part III, Item 12 of this Annual Report on Form 10-K, and is incorporated herein by reference.
Issuer Purchases of Equity Securities
The Company did not repurchase any of its equity securities during the fourth quarter ended December 31, 2023.
Use of Proceeds from the Sale of Registered Securities
On February 13, 2023, our Registration Statement, as amended, and originally filed on Form S-1 (File No. 333-267951) was declared effective by the SEC for our initial public offering of 1,317,647 units, including 197,647 additional common stock, tradeable warrants and/or non-tradeable warrants, by the underwriters pursuant to the exercise of the over-allotment option, each at an offering price of $6.48 per share, $0.01 per tradeable warrant, and/or $0.01 per non-tradeable warrant, for aggregate gross proceeds of approximately $8.4 million. After deducting underwriting discounts and commissions and other estimated offering expenses incurred by us of approximately $1.1 million, the net proceeds from the offering were approximately $7.3 million. WallachBeth Capital LLC acted as sole book-running manager and the representative of the underwriters of the initial public offering. No offering costs were paid or are payable, directly, or indirectly, to our directors or officers, to persons owning 10% or more of any class of our equity securities, or to any of our affiliates. Our common stock and tradeable warrants are traded on Nasdaq under the symbols “BFRG” and “BFRGW”, respectively.
| 23 |
There has been no material change in the expected use of the net proceeds from our IPO as described in our final prospectus filed with the SEC on February 16, 2023. Upon receipt, the net proceeds from our IPO were held in cash, cash equivalents and short-term investments. We initially used a portion of the net proceeds from the IPO, primarily on D&O Insurance, repayment of debt that was not converted in the IPO and accrued expenses for technology access, consultants and compensation. We also used and continue to use the proceeds for costs for operations. Pending such uses, we plan to continue investing the unused proceeds from the IPO in fixed, non-speculative income instruments and money market funds.
On February 5, 2024 the Company received net proceeds of approximately $4.9 million dollars from an underwritten public offering of 1,507,139 shares of common stock (or pre-funded warrants in lieu thereof) and accompanying warrants to purchase 1,507,139 shares of common stock at an offering price of $3.782. The 5 year warrants have an exercise price of $4.16. On February 21, 2024, the underwriters elected to exercise the over-allotment option for the purchase of an additional 218,382 shares of common stock, and the Company received additional net proceeds of approximately $750,000, pursuant to the exercise of the over-allotment.
ITEM 6. [RESERVED]
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OR PLAN OF OPERATION
The following discussion and analysis of the results of operations and financial condition of Bullfrog AI Holdings, Inc. (“Bullfrog”) as of and for the years ended December 31, 2023 and 2022 should be read in conjunction with our consolidated financial statements and the notes to those consolidated financial statements that are included elsewhere in this Annual Report. References in this Management’s Discussion and Analysis of Financial Condition and Results of Operations to “us”, “we”, “our” and similar terms refer to the Company. This Management’s Discussion and Analysis of Financial Condition and Results of Operations contains statements that are forward-looking. These statements are based on current expectations and assumptions that are subject to risk, uncertainties, and other factors. These statements are often identified by the use of words such as “may,” “will,” “expect,” “believe,” “anticipate,” “intend,” “could,” “estimate,” or “continue,” and similar expressions or variations. Actual results could differ materially because of the factors discussed in “Risk Factors” elsewhere in this Annual Report, and other factors that we may not know.
OVERVIEW
Bullfrog AI Holdings, Inc. was incorporated in the State of Nevada on February 6, 2020. Bullfrog AI Holdings, Inc. is the parent company of Bullfrog AI, Inc. and Bullfrog AI Management, LLC, which were incorporated in Delaware and Maryland, in 2017 and 2021, respectively. Operations are currently conducted through BullFrog AI Holdings, Inc., which began operations on February 6, 2020. We are a company focused specifically on advanced Artificial Intelligence / Machine Learning (AI/ML) analysis of complex data in the advancement of medicine. Our AI/ML platform (trade name: bfLEAP™) was created from technology originally developed at The Johns Hopkins University Applied Physics Laboratory (JHU-APL).
In February 2018, BullFrog AI Holdings secured the original exclusive, worldwide, royalty-bearing license from JHU-APL for the technology. The license covers three (3) issued patents, one (1) new provisional patent application, non-patent rights to proprietary libraries of algorithms and other trade secrets including modifications and improvements. We entered into a license agreement in July 2022 that provides the Company with new intellectual property and also encompasses most of the intellectual property from the February 2018 license. Our objective is to utilize our for a precision medicine approach toward drug development with biopharmaceutical collaborators, as well as our own internal clinical development programs. We believe the bfLEAP™ platform is ideally suited for evaluating pre-clinical and clinical trial data generated in translational research and clinical trial settings that lead to faster, less expensive drug approvals.
Our aim is to improve the odds of success in each stage of developing medicine, ranging from early pre-clinical through late-stage clinical development. Our ultimate objective is to utilize bfLEAP™ to enable the success of ongoing clinical trials or rescue late-stage failed drugs (i.e., Phase 2 or Phase 3 clinical trial failures) for development and divestiture; although, we will also consider collaborations for earlier stage drugs. We hope to accomplish this through strategic acquisitions of current clinical stage and failed drugs for in-house development, or through strategic partnerships with biopharmaceutical industry companies.
| 24 |
On July 8, 2022, the Company entered into an exclusive, worldwide, royalty-bearing license from JHU-APL for the additional technology. The new license provides additional intellectual property rights including patents, copyrights, and knowhow to be utilized under the Company’s bfLEAP™ analytical AI/ML platform. In consideration of the new license, the Company issued to JHU-APL 39,879 shares of common stock. In September 2020 and October of 2021, the Company executed amendments to the original license which represents improvements and new advanced analytics capabilities. In consideration of the rights granted to the Company under the original License Agreement, the Company granted JHU 178,571 warrants exercisable to purchase shares of common stock at $2.10 per share. Under the terms of the new License Agreement, JHU will be entitled to eight (8%) percent of net sales for the services provided by the Company to other parties and 3% for internally developed drug projects in which the JHU license was utilized. The new license also contains tiered sub licensing fees that start at 50% and reduce to 25% based on revenues. On May 31, 2023, the Company and JHU-APL entered into Amendment number 1 of the July 8, 2022 License Agreement whereby the Company gained access to certain improvements including additional patents and knowhow in exchange for a series of payments totaling $275,000. The first of these payments for $75,000 was due in July 2023 followed by annual payments of $75,000, $75,000 and $50,000 in years 2024, 2025 and 2026, respectively. The amendment also reduced the 2023 minimum annual royalty payment to $60,000, all other financial terms remain the same. As a result of this Amendment, the minimum annual payments are set to be $30,000 for 2022, $60,000 for 2023, and $300,000 for 2024 and beyond, all of which are creditable by royalties.
We intend to continue to evolve and improve bfLEAP™, either in-house or with development partners like JHU-APL. We plan to leverage our proprietary AI/ML platform developed over several years at one of the top innovation institutions in the world which has already been successfully applied in multiple sectors.
We have staffed our business using funds from our initial public offering and have entered into partnerships and relationships and recently completed our first commercial service contract with a leading rare disease non-profit organization for AI/ML analysis of late-stage clinical data. We have also acquired the rights to a series of preclinical and early clinical drug assets from universities, as well as a strategic collaboration with a world-renowned research institution to create a HSV1 viral therapeutic platform to engineer immunotherapies for a variety of diseases. We have signed exclusive worldwide License Agreements with JHU for a cancer drug that targets glioblastoma (brain cancer), pancreatic cancer, and others. We have also signed an exclusive worldwide license from George Washington University for another cancer drug that targets hepatocellular carcinoma (liver cancer) and other liver diseases. Additionally, we intend to gain access to later-stage clinical assets through partnerships or the acquisition of rights to failed therapeutic candidates for drug rescue. In certain circumstances, we intend to conduct late-stage clinical trials in an effort to rescue therapeutic assets that previously failed. In these cases, there will be a requirement for drug supply and regulatory services to conduct clinical trials. The success of our clinical development programs will require finding partners to support the clinical development, adequate availability of raw materials and/or drug product for our R&D and clinical trials, and, in some cases, may also require establishment of third-party arrangements to obtain finished drug product that is manufactured appropriately under (GMP) industry-standard guidelines, and packaged for clinical use or sale. Since we are a company focused on using our AI technology to advance medicines, any clinical development programs will also require, in all cases, partners and the establishment of third-party relationships for execution and completion of clinical trials.
Since completing our IPO on February 14, 2023, aided by the receipt of the IPO proceeds, we have initiated several initiatives: Investor relations and marketing to promote and raise awareness of the company in the financial and business sectors, research and development, collaboration with J Craig Venter Institute and in the quarter ended September 30, 2023, completed a preclinical study for our Mebendazole prodrug program. The Company is actively engaged in developing and seeking out new intellectual property as it strives to continuously evolve its AI/ML platform. Additionally, the Company has engaged a business development firm specializing in the biopharmaceutical industry to seek and secure a strategic development partner for our Mebendazole program.
| 25 |
Internally, the Company has added incremental staff to accelerate execution, and the development of processes and custom scripts for use in performing analytical services for customers, while also launching initiatives targeting large public health data sources and seeking access to proprietary health data sources. We also transitioned our accounting and financial reporting systems and processes to enhance our internal control environment as a public company. Capital from the IPO was also used to retire two notes that were sold to fund the Company through the IPO that did not convert into common stock as well as other debts accrued over time to our staff, employees and consultants as well as obligations related to the acquisition of our licensed drug programs.
In February 2024 the Company received net proceeds of approximately $4.9 million dollars from an underwritten public offering of 1,507,139 shares of common stock (or pre-funded warrants in lieu thereof) and accompanying warrants to purchase 1,507,139 shares of common stock at an offering price of $3.782. The 5 year warrants have an exercise price of $4.16. On February 21, 2024, the underwriters elected to exercise the over-allotment option for the purchase of an additional 218,382 shares of common stock, and the Company received additional net proceeds of approximately $750,000, pursuant to the exercise of the over-allotment. In the absence of significant revenues in 2024 the Company believes that its capital resources are sufficient to fund planned operations for more than 12 months from the date of this filing.
Our Strategy
The Company has a unique strategy designed to reduce risk and increase the frequency of cash flow. The first part of the strategy is to generate revenues through strategic relationships with biopharma companies. These relationships will be structured as a combination of fees and intellectual property based on the specific scope of the engagement. The objective of these engagements will be to uncover valuable insights to reduce the risk and/or increase the speed of the drug development process which can be achieved through manual or automated integration into the client’s workflow or analysis of discrete data sets.
In the future, the second part of our strategy involves acquiring the rights to clinical stage drugs, using our bfLEAP technology to design a precision medicine trial, conduct the trial with a partner, and sell the asset. This approach may also apply to earlier phases in the drug development process such as discovery and preclinical. In any case, the objective is to create near term value and exit and monetize as quickly as possible, preferably within approximately 30 months.
Results of Operations
For the years ended December 31, 2023 and 2022
Revenue and Costs of Goods Sold
We recognized $65,000 and $10,000 in revenue and $5,200 and $800 in costs of goods sold during the years ended December 31, 2023 and 2022, respectively.
| Year ended December 31, | Net Change | |||||||||||
| 2023 | 2022 | |||||||||||
| Operating expenses: | ||||||||||||
| Research and development | $ | 1,432,614 | $ | 609,270 | $ | 823,344 | ||||||
| General and administrative | 3,994,710 | 1,855,731 | 2,138,979 | |||||||||
| Total operating expenses | $ | 5,427,324 | $ | 2,465,001 | $ | 2,962,323 | ||||||
| 26 |
Research and Development
Our research and development expenses for the year ended December 31, 2023 increased by $823,344 compared to the same period ended December 31, 2022, primarily due to the inclusion of the cost of salaries and consulting fees in 2023 as we initiated our collaboration with J Craig Venter Institute and completion of a preclinical study for our Mebendazole prodrug program, as well as the cost of acquiring access to additional technology from JHU-APL related to bfLEAP™ pursuant to Amendment 1 of the July 2022 License Agreement. In 2022, the majority of the research and development expenses were directly related to the acquisition of two drug development product candidates including Mebendazole.
General and Administrative
Our general and administrative expenses for the year ended December 31, 2023 increased by $2,138,979, compared to the same period ended December 31, 2022, primarily due to higher salary and consulting costs reflecting an increased level of service as well the initiation of investor relations and marketing efforts and the transition of our accounting and financial reporting process to support a public company. The 2023 period also reflects approximately $120,000 in recruiting fees related to staff additions.
Other Income (Expense), Net
Interest expense decreased $268,056 for the year ended December 31, 2023, compared to the same period ended December 31, 2022 due to the majority of our debt converting or being paid off in the first quarter of 2023. The loss on the conversion of notes of $92,959 for the year ended December 31, 2023 was due to the conversion of the convertible notes. Other income increased by $183,244 due to interest earned on our IPO proceeds which we hold in an overnight sweep account.
Liquidity and Capital Resources
In 2022, the Company received net proceeds from the sale of Convertible Bridge Notes of approximately $1,016,000 and repaid the unsecured promissory notes sold in 2021 in the amount of $49,000. The Company sold one additional promissory note and received net proceeds of $100,000 in January 2023.
For the year ended December 31, 2022, the Company used approximately $911,000 on operating activities versus approximately $382,000 for the same period in 2021. The 2022 cash use included approximately $548,000 in salaries, approximately $634,000 in consulting and professional fees including legal, accounting and auditing fees, as well as consulting fees for operational activities and approximately $609,000 in technology license fees, patent cost reimbursements and minimum annual royalties which has been recorded as a research & development expense.
Through December 31, 2023, the Company has an accumulated deficit of approximately $9,755,000 and funded its operations through the sale of common stock and debt. We anticipate that our expenses will increase in the future to support our service offerings, clinical and pre-clinical research and development activities associated with strategic partnering and collaborations, as well as acquired product candidates and the increased costs of operating as a public company. These increases could include increased costs related to the hiring of additional personnel and fees to outside consultants, lawyers and accountants, among other expenses. Additionally, we anticipate increased costs associated with being a public company including expenses related to services associated with maintaining compliance with exchange listing and Securities and Exchange Commission requirements, insurance, and investor relations costs.
The Company’s current operations include BullFrog AI, Inc. and BullFrog Management, LLC, which are wholly owned subsidiaries of BullFrog AI Holdings, Inc., which is a holding company that depends upon the sale of its securities and cash generated through its subsidiaries to fund consolidated operations.
| 27 |
On February 16, 2023, the Company completed its IPO of 1,297,318 units (each, a “Unit,” collectively, the “Units”) at a price of $6.50 per unit for a total of approximately $8.4 million of gross proceeds to the Company. Each Unit consists of one share of the Company’s common stock, one tradeable warrant (each, a “Tradeable Warrant,” collectively, the “Tradeable Warrants”) to purchase one share of common stock at an exercise price of $7.80 per share, and one non-tradeable warrant (each, a “Non-tradeable Warrant,” collectively, the “Non-tradeable Warrants”; together with the Tradeable Warrants, each, a “Warrant,” collectively, the “Warrants”) to purchase one share of the Company’s common stock at an exercise price of $8.125. In connection with the IPO, the Company also completed a 1-for-7 reverse stock split of our common stock.
In connection with the IPO, a SAFE and convertible loan agreement held by a related party converted into 55,787 shares of post reverse split common stock. Additionally, all outstanding convertible bridge notes and accrued interest through November 30, 2022 were converted into 276,289 shares of common stock and 276,289 warrants to purchase common stock were issued to the Convertible Bridge Note holders at conversion. The convertible bridge note conversions and the warrant exercise pricing were determined using a $25 million dollar company valuation immediately before the IPO.
Between April 5 and April 13, 2023, the holders of warrants exercised 436,533 warrants for common stock at various exercise prices and the Company received proceeds of approximately $1,495,000.
In the absence of revenues in 2024 management believes the company’s capital resources are sufficient to fund planned operations for substantially longer than 12 months from the date of this filing.
Consolidated Cash Flow Data
| Year ended December 31, | Net Change | |||||||||||
| 2023 | 2022 | |||||||||||
| Net cash (used in) provided by | ||||||||||||
| Research and development Operating activities | $ | (6,001,299 | ) | $ | (910,890 | ) | $ | (5,090,409 | ) | |||
| General and administrative Investing activities | - | (8,744 | ) | 8,744 | ||||||||
| Financing activities | 8,568,359 | 967,290 | 7,601,069 | |||||||||
| Net increase in cash and cash equivalents | $ | 2,567,060 | $ | 47,656 | $ | 2,519,404 | ||||||
Cash Flows Used in Operating Activities
Net cash used in operating activities for the year ended December 31, 2023 increased by $5,090,409 compared to the same period ended December 31, 2022 primarily due to paying down accrued expenses for technology access, consultants, and compensation in 2023, coupled with increased operating costs, including D&O insurance premiums.
Cash Flows Used in Investing Activities
There was no cash used in investing activities during the year ended December 31, 2023.
Cash Flows Provided by Financing Activities
Net cash provided by financing activities for the year ended December 31, 2023 increased by $7,601,069, compared to the same period ended December 31, 2022 primarily due to the completion of our Initial Public Offering in February 2023 and proceeds received pursuant to warrant exercises.
| 28 |
Critical Accounting Policies
In Footnote 2 of our Audited Financial Statements for the year ended December 31, 2023 found elsewhere in this filing, we included a discussion of the most critical accounting policies used in the preparation of our financial statements. There has been no material change in the policies and estimates used in the preparation of our financial statements since the completion of the 2023 audit.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, as such term is defined in Item 303(a)(4) of Regulation S-K.
Financial Operations Overview
Revenue
While we generated our first revenues in late 2022 from our services provided to a pharmaceutical customer, in the third quarter of 2023 we completed our first commercial service contract and recognized revenue in the amount of $65,000. We have service contracts with two organizations and currently have multiple discussions underway, although there can be no assurance of entering into additional service agreements and business relationships in 2024.
Operating Expenses
We classify our operating expenses into two categories: research and development and general and administrative. Prior to 2022, most of our activities were related to: technology evaluation, acquisition and validation, capital acquisition and business development activities in general, which we believe have readied the Company for contract services while exploring strategic partnering and asset acquisition. These activities and related expenditures have been recorded and reported as General and Administrative in our Financial Statements. In 2022, we licensed two drug development programs from universities and also entered into a new license with JHU-APL for new IP and other enhancements used with our bfLEAP™ platform. In 2022, we expended appropriately $608,000 on license related payments for our bfLEAP™ AI/ML platform and our two drug development programs from universities. We expect that our research and development expenses will increase in 2024 as we initiate activities directed towards the development of service offering products, collaborations (JCVI) and preclinical studies aimed at generating the data to enable the filing of an Investigational New Drug (IND) application.
Research and Development Costs and Expenses
Research and development costs and expenses in 2022 consisted primarily of costs related to the acquisition of licensed technology. In 2023 we have initiated development activities on our licensed drug candidates and our discovery collaboration with JCVI. In addition to fees paid to external service providers, we are also allocating internal costs for personnel working on these efforts in addition to personnel costs related to our internal efforts to develop our product and service offerings using bfLEAP™. We anticipate our research and development costs could become significant as we execute on our business plan and begin conducting preclinical research and development activities directed at securing development partners and filing an IND for our licensed drug development programs described in this filing, as well as under strategic partnerships and for other drug development programs we may acquire. Research and development expenses are recorded in operating expenses in the period in which they are incurred. Estimates will be used in determining the expense liability of certain costs where services have been performed but not yet invoiced. We will monitor levels of performance under each significant contract for external services through communications with the service providers to reflect the actual amount expended.
| 29 |
General and Administrative Expenses
In anticipation of the IPO, a management team with deep industry experience was identified and engaged as employees and consultants to assist the Company in preparing for the IPO and subsequently, to operate and function as a public company. Through 2022, the primary activities included: technology evaluation, acquisition, and validation, capital acquisition and business development activities which in general, have readied the Company for contract services while exploring strategic partnering and asset acquisition as noted above. In February 2023, the Company achieved its objective of completing an IPO and listing on NASDAQ. Our 2023 general and administrative expenses are significantly higher than our 2022 general and administrative expenses due to several factors. The primary increases in 2023 relate to new costs associated with being a public company such as D&O insurance, professional services engaged to support SEC compliance as well as higher salary and consulting expenses as we have hired additional staff and consultants. We have also increased our business development, investor relations and marketing efforts. We anticipate that our general and administrative expenses may increase in the future to support our service offerings, clinical and pre-clinical research and development activities associated with strategic partnering and collaborations.
Emerging Growth Company and Smaller Reporting Company Status
The Company is an emerging growth company as defined in the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”) and may take advantage of reduced reporting requirements that are otherwise applicable to public companies. Section 107 of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies are required to comply with those standards. We have elected to use the extended transition period to comply with new or revised accounting standards. This may make it difficult to compare our financial results with the financial results of another public company that is either not an emerging growth company or is an emerging growth company that has chosen not to take advantage of the extended transition period exemptions because of the potential differences in accounting standards used.
We are also a “smaller reporting company”, meaning that the market value of our stock held by non-affiliates plus the aggregate amount of gross proceeds to us as a result of the IPO is less than $700 million and our annual revenue was less than $100 million during the most recently completed fiscal year. We may continue to be a smaller reporting company if either (i) the market value of our stock held by non-affiliates is less than $250 million or (ii) our annual revenue was less than $100 million during the most recently completed fiscal year and the market value of our stock held by non-affiliates is less than $700 million. If we are a smaller reporting company at the time we cease to be an emerging growth company, we may continue to rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and, similar to emerging growth companies, smaller reporting companies have reduced disclosure obligations regarding executive compensation.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As a smaller reporting company, this disclosure is not required.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements required to be filed pursuant to this Item 8 are appended to this Annual Report on Form 10-K. See “Index to Consolidated Financial Statements” which appears on page F-1 of this Annual Report on Form 10-K, and is incorporated herein by reference.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
| 30 |
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We are transitioning to and will maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized, and timely reported as provided in SEC rules and forms and that such information is accumulated and communicated to our management, as appropriate, to allow for timely decisions regarding required disclosure. We will periodically review the design and effectiveness of our disclosure controls and procedures, including compliance with various laws and regulations that apply to our operations. We will make modifications to improve the design and effectiveness of our disclosure controls and procedures and may take other corrective action if our reviews identify a need for such modifications or actions. In designing and evaluating the disclosure controls and procedures, we recognize that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and we will apply judgment in evaluating the cost-benefit relationship of possible controls and procedures. In addition, the design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, controls may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a control system, misstatements due to error or fraud may occur and not be detected.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during the fiscal year ended December 31, 2023 which have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting
This Annual Report does not include a report of management’s assessment regarding internal control over financial reporting due to a transition period established by the rules of the SEC for newly public companies.
Attestation Report of Independent Registered Public Accounting Firm
This Annual Report does not include an attestation report of our registered independent public accounting firm regarding internal control over financial reporting due to an exemption established by the JOBS Act for “emerging growth companies.”
ITEM 9B. OTHER INFORMATION
None.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
| 31 |
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS, AND CORPORATE GOVERNANCE
Executive Officers and Directors
The following table sets forth the name, age and position of each of our executive officers, key employees and directors.
| Name | Age | Position(s) | ||
| Executive Officers: | ||||
| Vin Singh | 55 | Chief Executive Officer and Director | ||
| Dane Saglio | 66 | Chief Financial Officer | ||
| Non-Executive Directors: | ||||
| Don Elsey | 70 | Director and Chair Audit Committee | ||
| William Enright | 60 | Director and Chair of Compensation Committee | ||
| Jason Hanson | 54 | Director and Chair of Nominating and Corporate Governance Committee |
Vininder (Vin) Singh is the Founder, Chairman, and CEO of BullFrog AI Holdings, Inc. since its inception in August 2017. Over the past five years, he has built the Company from scratch and during that time he led strategy, built a highly experienced team of leaders, spear headed the acquisition and development of BullFrog’s core AI technology and drug assets, secured the first revenue, and raised approximately $2M in financing. In February of 2020, he formed BullFrog AI Holdings, Inc. and BullFrog AI Inc. became a wholly owned subsidiary designated as the holder of core intellectual property. Vin is a serial entrepreneur and experienced executive with 25 years of experience in the life sciences and biotechnology industries. He has extensive start-up experience having founded and built several pioneering investor backed companies including BullFrog AI, which uses machine learning/AI to enable drug development, Next Healthcare Inc., a personalized diagnostics and adult cell banking service, and MaxCyte Inc. (MXCT), a cell therapy company. He was also an executive at GlobalStem Inc. and ThermoFisher Scientific, leading their global cell therapy services business. Vin has a BS in Electrical Engineering from Rutgers University, an MS in Biomedical Engineering from Rensselaer Polytechnic Institute, and an MBA from Johns Hopkins University. We believe that Mr. Singh is qualified to serve as a member of our board of directors due to the perspective and experience that he brings as our Founder and Chief Executive Officer, his extensive experience in the science and biotechnology industries and in the management of startup companies.
Dane Saglio joined BullFrog Holdings AI, Inc. as Chief Financial Officer in September 2021. Mr. Saglio brings more than 40 years of financial management experience in both public and private companies across a number of business sectors. Previously, Mr. Saglio has served as CFO at Seneca Biopharma, RegeneRx Biopharmaceuticals since 2011, New Generation Biofuels 2010 until 2011, and EntreMed from 2000 until 2008, all public companies in the biotechnology arena. Prior to joining the Company, Mr. Saglio was the CFO of Seneca Biopharma, initially as a consultant in August 2019 and then as an employee in April 2020 until the Company merged with Leading Bio Sciences, forming Palisades Bio, Inc. in April 2021. He previously served as CFO at Celios Corporation from October 2017 until July 2019 and Helomics Corporation, a personalized medicine company in cancer from October 2014 through July 2017. He began his career at Informatics Corp, now Computer Associates International and then at Bressler & Reiner, a DC-based real estate developer and homebuilder. Dane has a BS from the University of Maryland is a licensed CPA in Maryland (inactive).
R. Don Elsey has been a director and chair of the Audit Committee of our board since February 14, 2023. Currently, Mr. Elsey is the Audit Chair of OpGen, Inc., a precision medicine company. Mr. Elsey was the CFO of Lyra until his retirement in December 2020. Previously, from February 2015 to February 2019, Mr. Elsey served as Chief Financial Officer at Senseonics, Inc., a medical device company. From May 2014 until February 2015, Mr. Elsey served as Chief Financial Officer of Regado Biosciences, Inc., a biopharmaceutical company. From December 2012 to February 2014, Mr. Elsey served as Chief Financial Officer of LifeCell Corporation, a privately held regenerative medicine company. Mr. Elsey holds a B.A. in economics and an M.B.A. in finance from Michigan State University. We believe that Mr. Esley is qualified to serve as a member of our board of directors because of his extensive professional experience in science and biotechnology companies.
| 32 |
William “Bill” Enright has been a director and chair of the Compensation Committee of our board since February 14, 2023. He is a seasoned biotech executive with more than thirty-four years of experience in building and financing both privately held and publicly held companies and He is currently the CEO and a Director of Barinthus Biotherapurtics plc (NASDAQ: BRNS), which he helped to take public in April 2021. Prior to Barinthus, Bill spent more than ten years at Altimmune (NASDAQ: ALT) as a Director, President & CEO, moving multiple programs into clinical testing, completing several acquisitions, and eventually taking the company public. Prior to joining Altimmune, Bill spent six years with GenVec, Inc. (acquired by Precigen) with increasing responsibilities, culminating as Head of Business Development. Bill brings a breadth of experiences in a variety of positions within the life science/biotech industry, including time as a consultant, a bench scientist and 12 years with Life Technologies, Inc. (acquired by Thermo-Fisher), working in various senior level licensing, business management, manufacturing and research roles. Bill received a Master of Arts in Molecular Biology from SUNY at Buffalo and a Master of Science in Business Management from Johns Hopkins University. We believe that Mr. Enright is qualified to serve as a member of our board of directors because of his extensive professional experience in life science/biotech companies and in the management of public companies.
Jason Hanson has served as a director and chair of the Nominating and Corporate Governance Committee since February 14, 2023. Mr. Hanson has served as Chief Executive Officer and as a Director of enGene Inc. since July 2018. He also served as President of enGene Inc. from July 2018 to December 2022. Mr. Hanson effectively re-launched enGene from a small private company working in the GI discovery space into a clinical stage gene therapy oncology company trading on Nasdaq, implementing a new scientific, technical and strategic vision for the Company. From August 2016 to November, 2017, Mr. Hanson served as President and Chief Executive Officer of Ohana Biosciences, a biotechnology company based in Cambridge, MA, and as member of the Ohana Board of Directors and consultant to Ohana from November 2017 to June 2018. Mr. Hanson previously served as Executive Vice President and Chief Strategy Officer for NuVasive, Inc. from November 2015 to August 2016. Mr. Hanson served as Corporate Vice President of General Electric Company and member of the senior executive team of GE Healthcare, a global pharmaceutical, medical device and healthcare services business from May 2014 to October 2015. In January 2013, Mr. Hanson served as Company Group Chairman and Executive Vice President of Valeant Pharmaceuticals International, Inc. (now Bausch Health Companies Inc.). Previously, he served in various roles at Medicis Pharmaceutical Corporation, including as Executive Vice President and Chief Operating Officer between July 2006 and December 2012. Mr. Hanson also served in numerous roles at GE Healthcare, including General Counsel roles, from April 1999 to July 2006. Mr. Hanson holds a B.S. from Cornell University and a J.D. from Duke University School of Law.
Board Diversity
The table below provides certain information regarding the diversity of our board of directors as the date of this annual report.
| Board Diversity Matrix | ||||
| Country of Principal Executive Offices: | United States | |||
| Foreign Private Issuer | No | |||
| Disclosure Prohibited under Home Country Law | N/A | |||
| Total Number of Directors | 4 | |||
| Female | Male | Non-Binary | Did Not Disclose Gender | |
| Part I: Gender Identity | ||||
| Directors | 0 | 4 | 0 | 0 |
| Part II: Demographic Background | ||||
| Underrepresented Individual in Home Country Jurisdiction | N/A | |||
| LGBTQ+ | [*] | |||
| Did Not Disclose Demographic Background | [*] | |||
| 33 |
Our Board seeks members from diverse professional backgrounds who combine a solid professional reputation and knowledge of our business and industry with a reputation for integrity. Our Board does not have a formal policy concerning diversity and inclusion but is in the process of establishing a policy on diversity. Diversity of experience, expertise, and viewpoints is one of many factors the Nominating and Corporate Governance Committee considers when recommending director nominees to our Board. Further, our Board is committed to actively seeking highly qualified women and individuals from minority groups and the LGBTQ+ community to include in the pool from which new candidates are selected. Our Board also seeks members that have experience in positions with a high degree of responsibility or are, or have been, leaders in the companies or institutions with which they are, or were, affiliated, but may seek other members with different backgrounds, based upon the contributions they can make to our Company. While the Board has continued its efforts to identify candidates that have such experience, they have currently been unable to identify any such candidates which fulfill the diversity requirement with the requisite professional experience.
Role of Board of Directors in Risk Oversight Process
The board of directors has extensive involvement in the oversight of risk management related to us and our business and accomplishes this oversight through the regular reporting by the Audit Committee. The information set forth in Item 1C is incorporated herein by reference.
Director Independence
Messrs. Elsey, Enright and Hanson, three members of our Board of Directors, are independent using the definition of independence under Nasdaq Listing Rule 5605(a)(2) and the standards established by the SEC.
Committees of our Board
Audit Committee
Our audit committee consists of Don Elsey, William Enright and Jason Hanson, with Mr. Elsey serving as chair. Our board of directors has affirmatively determined that each meets the definition of “independent director” under the rules of The Nasdaq Capital Market, and that they meet the independence standards under Rule 10A-3. Each member of our audit committee meets the financial literacy requirements of Nasdaq rules, and qualify as a financial expert within the meaning of SEC regulations and meets the financial sophistication requirements of the pertinent listing standards of Nasdaq, as in effect from time to time. In making this determination, our board of directors has considered the members’ formal education and previous and current experience in financial roles. Our board of directors has adopted a written charter for the audit committee, which can be found on our website at https://ir.bullfrogai.com/corporate-governance/governance-documents.
The audit committee is appointed by the board of directors to assist the board of directors in its duty to oversee the Company’s accounting, financial reporting, and internal control functions and the audit of the Company’s financial statements. The role of the audit committee is to oversee management in the performance of its responsibility for the integrity of the Company’s accounting and financial reporting and its systems of internal controls, the performance and qualifications of the Company’s independent auditor, including the independent auditor’s independence, the performance of the Company’s internal audit function; and the Company’s compliance with legal and regulatory requirements. The Audit Committee met four times in 2023.
| 34 |
Compensation Committee
Our compensation committee consists of William Enright, Don Elsey and Jason Hanson, with Mr. Enright serving as chair. Our board of directors has adopted a written charter for the compensation committee, which can be found on our website at https://ir.bullfrogai.com/corporate-governance/governance-documents.
The compensation committee is responsible for reviewing and recommending, among other things:
| ● | the adequacy and form of compensation of the board; | |
| ● | the compensation of Chief Executive Officer, including base salary, incentive bonus, stock option and other grant, award and benefits upon hiring and on an annual basis; | |
| ● | the compensation of other senior management upon hiring and on an annual basis; and | |
| ● | the Company’s incentive compensation and other equity-based plans and recommending changes to such plans to our board of directors, when necessary. |
Nominating & Corporate Governance Committee
Our nominating and corporate governance committee consists of Jason Hanson, William Enright and Don Elsey, with Mr. Hanson serving as chair. Our board of directors has adopted a written charter for the nominating and corporate governance committee, which can be found on our website at https://ir.bullfrogai.com/corporategovernance/governance-documents.
The nominating committee is responsible for, among other things:
| ● | developing criteria for membership on the board of directors and committees; | |
| ● | identifying individuals qualified to become members of the board of directors; | |
| ● | recommending persons to be nominated for election as directors and to each committee of the board of directors; | |
| ● | annually reviewing our corporate governance guidelines; and | |
| ● | monitoring and evaluating the performance of the board of directors and leading the board in an annual self-assessment of its practices and effectiveness. |
Term of office
All directors hold office until the next annual meeting of the stockholders of the company and until their successors have been duly elected and qualified. Officers are elected by and serve at the discretion of our Board.
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, employees or persons performing similar functions. Our code of ethics can be found at https://ir.bullfrogai.com/corporate-governance/governance-documents.
Clawback Policy
On December 1, 2023, the Board adopted the BullFrog AI Clawback Policy (the “Clawback Policy”), effective December 1, 2023, providing for the recovery of certain incentive-based compensation from current and former executive officers of the Company in the event the Company is required to restate any of its financial statements filed with the SEC under the Exchange Act in order to correct an error that is material to the previously-issued financial statements, or that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period. Adoption of the Clawback Policy was mandated by new Nasdaq listing standards introduced pursuant to Exchange Act Rule 10D-1. The Clawback Policy is in addition to Section 304 of the Sarbanes-Oxley Act of 2002 which permits the SEC to order the disgorgement of bonuses and incentive-based compensation earned by a registrant issuer’s chief executive officer and chief financial officer in the year following the filing of any financial statement that the issuer is required to restate because of misconduct, and the reimbursement of those funds to the issuer. A copy of the Clawback Policy has been filed herewith, and can also be found at www.bullfrogai.com.
| 35 |
Family Relationships
There are no family relationships among and between the issuer’s directors, officers, persons nominated or chosen by the issuer to become directors or officers, or beneficial owners of more than ten percent of any class of the issuer’s equity securities.
Involvement in Certain Legal Proceedings
Our directors and executive officers have not been involved in any of the following events during the past ten years:
| 1. | any bankruptcy petition filed by or against such person or any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; | |
| 2. | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); | |
| 3. | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from or otherwise limiting his involvement in any type of business, securities or banking activities or to be associated with any person practicing in banking or securities activities; | |
| 4. | being found by a court of competent jurisdiction in a civil action, the SEC or the Commodity Futures Trading Commission to have violated a Federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; | |
| 5. | being subject of, or a party to, any Federal or state judicial or administrative order, judgment decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of any Federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or | |
| 6. | being subject of or party to any sanction or order, not subsequently reversed, suspended, or vacated, of any self-regulatory organization, any registered entity or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Section 16(a) Beneficial Ownership Compliance
Based solely upon a review of copies of such forms filed on Forms 3, 4 and 5, and amendments thereto furnished to us, we believe that as of the date of this Report, our executive officers, directors and greater than 10 percent beneficial owners have complied on a timely basis with all Section 16(a) filing requirements, except Messrs. Elsey, Enright and Hanson did not file Form 3s upon their appointment to the Board.
Nomination Process
As of December 31, 2023, we did not affect any material changes to the procedures by which stockholders may recommend nominees to the Board of Directors.
Insider Trading Policies
We have adopted an insider trading policy governing the purchase, sale, and other dispositions of our securities by directors, senior management, and employees. A copy of the insider trading policy is attached as an exhibit to this annual report.
| 36 |
ITEM 11. EXECUTIVE COMPENSATION
Summary Compensation Table
The following table sets forth all plan and non-plan compensation for the last two fiscal years paid to individuals who served as the Company’s principal executive officers and the Company’s two other most highly compensated executive officers serving as executive officers at the end of the last completed fiscal year, as required by Item 402(m)(2) of Regulation S-K of the Securities Act. We refer to these individuals collectively as our “named executive officers.”
| Name and Principal Position | Year | Salary | Bonus | Stock Awards | Option Awards | All Other Compensation | Nonequity Incentive Plan Compensation | Nonqualified Deferred Compensation Earnings | Total Compensation | |||||||||||||||||||||||||||
| Vininder Singh | 2023 | $ | 707,666 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | 707,666 | |||||||||||||||||||
| Chief Executive Officer and Director | 2022 | $ | 179,000 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | 179,000 | |||||||||||||||||||
| Dane Saglio | 2023 | $ | 310,000 | $ | 50,000 | $ | - | $ | 147,000 | $ | - | $ | - | $ | - | $ | 507,000 | |||||||||||||||||||
| Chief Financial Officer | 2022 | $ | 30,000 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | 30,000 | |||||||||||||||||||
Employment Agreements
On May 16, 2022, we entered into an employment agreement with Vininder Singh, pursuant to which he will receive received an annual base salary of $400,000, which is subject to bi-annual review by the Company. Mr. Singh will also be eligible for an annual bonus based on the achievement of certain goals and performance criteria established by the Board. Mr. Singh’s target annual bonus for the fiscal years ended 2022 through 2025 will be a minimum of twenty (20%) percent of the current base salary, with a maximum payout of up to one-hundred (100%) percent based on target achievement. For 2023, the criteria to determine Mr. Singh’s bonus will include the following: (i) the Company achieves $500,000 in sales; (ii) the filing of an Investigational New Drug (IND) Application with the FDA for mebendazole; (iii) the Company enters into two (2) strategic partnerships; and (iv) the Company commences partner negotiations with a third party for HSV-1, bf-114 or bf-222. Mr. Singh will also be eligible to participate in the Company’s stock incentive plan, subject to Board approval. The agreement with Mr. Singh shall continue until either his resignation, termination for cause by the Company, or death or disability of Mr. Singh.
Director Compensation
The following table summarizes the compensation paid to our executive and non-executive directors during the year ended December 31, 2023.
| Name | Fees Earned or Paid in Cash (1) | Stock Awards | Option Awards (2) | All Other Compensation | Nonequity Incentive Plan Compensation | Nonqualified Deferred Compensation Earnings | Total Compensation | |||||||||||||||||||||
| Vininder Singh(3) | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||||
| Don Elsey | $ | 39,375 | $ | - | $ | 197,200 | $ | - | $ | - | $ | - | $ | 236,575 | ||||||||||||||
| William Enright | $ | 39,375 | $ | - | $ | 197,200 | $ | - | $ | - | $ | - | $ | 236,575 | ||||||||||||||
| Jason Hanson | $ | 39,375 | $ | - | $ | 197,200 | $ | - | $ | - | $ | - | $ | 236,575 | ||||||||||||||
(1) Represents cash compensation for service as a director and as chair of a board committee during the fiscal year 2023.
(2) Represents annual value of stock options issued during fiscal year 2023 under our 2022 Equity Incentive Plan.
(3) Mr. Singh did not receive additional compensation for his service as a director of our Company during the fiscal year 2023.
| 37 |
Pension, Retirement or Similar Benefit Plans
There are no arrangements or plans in which we provide pension, retirement or similar benefits for directors or executive officers. We have no material bonus or profit-sharing plans pursuant to which cash or non-cash compensation is or may be paid to our directors or executive officers, except that stock options may be granted at the discretion of the Board or a committee thereof.
Indebtedness of Directors, Senior Officers, Executive Officers and Other Management
None of our directors, executive officers or any associate or affiliate of our Company during the last two fiscal years is or has been indebted to our Company by way of guarantee, support agreement, letter of credit or other similar agreement or understanding currently outstanding.
Equity Compensation Plans
On November 30, 2022, our Board of Directors and shareholders adopted the 2022 Equity Incentive Plan (the “Plan”). Pursuant to the Plan, we are authorized to grant options and other equity awards to officers, directors, employees and consultants. The exercise price of each share of common stock purchasable under an award issued pursuant to the Plan, shall be determined by our compensation committee, in its sole discretion, at the time of grant, but shall not be less than 100% of the fair market of such share of common stock on the date the award is granted, subject to adjustment and conditions further described in the Plan. Our compensation committee shall also have sole authority to set the terms of all awards at the time of grant. As of December 31, 2023, there are 441,500 shares available under the Plan.
Outstanding Equity Awards at Fiscal Year-End
The following table summarizes the outstanding equity awards held by each named executive officer as of December 31, 2023. This table includes unexercised and unvested options and equity awards.
| Outstanding Equity Awards as of December 31, 2023 | ||||||||||||||||||||
| Option Awards | ||||||||||||||||||||
| Name | Date of Grant | Number of securities underlying unexercised options (#) exercisable | Number of securities underlying unexercised options (#) unexerciseable | Equity incentive plan awards: Number of securities underlying unexercised unearned options (#) | Option exercise price ($) | Option expiration date | ||||||||||||||
| Dane Saglio | March 17, 2023 | 43,750 | 31,250 | - | $ | 2.80 | March 17, 2033 | |||||||||||||
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information regarding the beneficial ownership of our common stock as of March 27, 2024 by:
| ● | each of our named executive officers; | |
| ● | each of our directors; | |
| ● | all of our current directors and named executive officers as a group; and | |
| ● | each stockholder known by us to own beneficially more than 5% of our common stock. |
| 38 |
Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities. Shares of common stock that may be acquired by an individual or group within 60 days of March 27, 2024, pursuant to the exercise of options or warrants, vesting of common stock or conversion of convertible debt, are deemed to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table. Percentage of ownership is based on 7,850,550 shares of common stock issued and outstanding as of March 27, 2024.
Except as otherwise indicated, all shares are owned directly. Unless otherwise indicated, the address of each of the persons shown is c/o Bullfrog AI Holdings, Inc., 325 Ellington Blvd., Unit 317, Gaithersburg, MD 20878.
| Name of Beneficial Owner | Common Stock Beneficially Owned | Percentage of Common Stock | ||||||
| Directors and Officers: | ||||||||
| Vininder Singh Chief Executive Officer and Director (1) | 2,618,779 | 33.25 | % | |||||
| Dane Saglio Chief Financial Officer(2) | 112,818 | 1.43 | % | |||||
| R. Don Elsey (3) | 23,332 | - | ||||||
| William Enright (3) | 23,332 | - | ||||||
| Jason Hanson (3) | 23,332 | - | ||||||
| All officers and directors as a group (5 persons) | 2,801,593 | 34.97 | % | |||||
| Beneficial owners of more than 5% | ||||||||
| Tivoli Trust (4) | 904,391 | 10.40 | % | |||||
| ● | Less than 1% |
| (1) | Comprised of 2,592,446 shares of Common Stock and 26,333 Stock Options exercisable within 60 days. |
| (2) | Comprised of 47,142 shares of Common Stock and 65,676 Stock Options exercisable within 60 days. Comprised of 23,332 Stock Options exercisable within 60 days. |
| (4) | Comprised of 73,449 shares of non-voting Series A Preferred Stock, 115,185 warrants exercisable at $2.50 per shares and 54,714 shares of Common Stock. Assumes the conversion of all Series A Preferred Stock into common stock in an amount equal to ten shares of common stock for each one share of Series A Preferred Stock. |
Securities Authorized for Issuance under Equity Compensation Plans
General. In November 2022, our Board of Directors adopted our 2022 Equity Incentive Plan (the “2022 Plan”) and the 2022 Plan was submitted to our stockholders for approval. Our 2022 Plan became effective immediately on adoption. Our 2022 Plan replaces our previous incentive plan. However, awards outstanding under our previous incentive plan will continue to be governed by their existing terms.
| 39 |
Share Reserve. The number of shares of our common stock available for issuance under our 2022 Plan is 900,000 shares. Notwithstanding the number of shares available for issuance, on the first day of each month commencing January 1, 2023, or the first business day of the calendar year if the first day of the calendar year falls on a Saturday or Sunday, the number of shares eligible for awards under the 2022 Plan will automatically increase in an amount equal to 15% of the total number of shares of common stock outstanding as of December 31st of the preceding fiscal year.
| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) | Weighted-average exercise price of outstanding options, warrants and rights (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a) | |||||||||
| Equity compensation plans approved by security holders | $ | |||||||||||
| Equity compensation plans not approved by security holders | 69,217 | $ | 3.06 | $ | ||||||||
| Total | 69,217 | $ | 3.06 | $ | ||||||||
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Other than as set forth below and compensation arrangements, including employment, and indemnification arrangements, discussed, there have been no transactions since January 1, 2021, in which the amount involved in the transaction exceeded or will exceed the lesser of $120,000 or one percent of the average of our total assets as at the year-end for the last two completed fiscal years, and to which any of our directors, executive officers or beneficial holders of more than 5% of our capital stock, or any immediate family member of, or person sharing the household with, any of these individuals, had or will have a direct or indirect material interest.
On July 8, 2021, the Company entered into a Simple Agreement for Future Equity (SAFE), with a related party, Tivoli Trust, our second largest shareholder (the “Investor”), with an amount of $150,000, with 0% interest. Under the SAFE agreement, if there is an Equity Financing before the termination of this SAFE, on the initial closing of such Equity Financing, this SAFE will automatically convert into the number of shares of SAFE Preferred Stock equal to the Purchase Amount divided by the Conversion Price, which means either: (1) the Safe Price (the price per share equal to the Post-Money Valuation Cap divided by the Company Capitalization) or (2) the Discount Price (the price per share of the Standard Preferred Stock sold in the Equity Financing multiplied by the Discount Rate), whichever calculation results in a greater number of shares of Safe Preferred Stock.
If there is a Liquidity Event before the termination of this SAFE, this SAFE will automatically be entitled (subject to the liquidation priority set forth in Section 1(d) below) to receive a portion of Proceeds, due and payable to the Investor immediately prior to, or concurrent with, the consummation of such Liquidity Event, equal to the greater of (i) the Purchase Amount (the “Cash-Out Amount”) or (ii) the amount payable on the number of shares of Common Stock equal to the Purchase Amount divided by the Liquidity Price (the “Conversion Amount”). If any of the Company’s securityholders are given a choice as to the form and amount of Proceeds to be received in a Liquidity Event, the Investor will be given the same choice, provided that the Investor may not choose to receive a form of consideration that the Investor would be ineligible to receive as a result of the Investor’s failure to satisfy any requirement or limitation generally applicable to the Company’s securityholders, or under any applicable laws.
This SAFE will automatically terminate (without relieving the Company of any obligations arising from a prior breach of or non-compliance with this SAFE) immediately following the earliest to occur of: (i) the issuance of Capital Stock to the Investor pursuant to the automatic conversion of this SAFE under agreement; or (ii) the payment, or setting aside for payment, of amounts due the Investor pursuant to the agreement.
| 40 |
As of December 31, 2021, the $150,000 received from SAFE was recorded at 6% imputed interest. The maturity date of the loan is defined by the SAFE agreement as discussed above. The SAFE was converted into 32,967 shares of common stock (post reverse stock split) upon the Company’s IPO in February 2023.
On August 19, 2021, the company entered into a convertible loan agreement with a related party, with a principal balance of $99,900 at 9% interest. The noteholder has the right to convert the principal and interest into common shares of the Company. This loan included an original issuance discount of 5% and included 99,900 Warrants at an exercise price of $1, exercisable for 5 years from the issue date on the face of the Warrant. The maturity date of the loan was February 19, 2022. In May 2022, the Company and the note holder agreed to cancel and void previous warrants and entered into a new agreement for 115,185 warrants with an exercise price of $2.50. As of December 31, 2022, the $99,900 principal and the $4,950 overpayment of the note remained outstanding and had accrued interest of $12,463. The warrants discussed above were initially discounted against the notes, subsequent to year end December 31, 2021, they were deemed voided and new warrants in accordance with the new terms were issued. We assessed the differences in fair value and determined that they were de minimis and expensed the full value of the new warrants. The noteholder elected to convert the loan into 21,747 shares of common stock (post reverse stock split) upon the Company’s IPO in February 2023.
On June 15, 2021, the company entered into a unsecured short term loan agreement with the Investor for an aggregate principal balance of $34,000, with a one-year maturity date, accruing interest at 5% and imputing an additional 1% interest.
On November 19, 2021, 2021, the company entered into an unsecured short term loan agreement with the Investor for an aggregate principal balance of $5,000, with a one-year maturity date, accruing interest at 5% and imputing an additional 1% interest.
On December 13, 2021, the company entered into an unsecured short term loan agreement with the Investor for an aggregate principal balance of $10,000, with a one-year maturity date, accruing interest at 5% and imputing an additional 1% interest.
On October 5, 2022, the Company entered into an exchange agreement with the Investor whereby all of his common stock, 734,493 shares of common stock (post reverse split shares), were exchanged into 73,449 shares of Series A Convertible Preferred Stock that converts to common at a rate of 10 common for one preferred. The Series A Preferred Stock is the economic equivalent of the common stock but has no voting rights and is subject to a blocker which prohibits the conversion into common stock if it would result in the Investor owning more than 4.99% of the Company’s outstanding common stock at such time. For a description of the rights and preferences of the Series A Preferred Stock, see “Description of Securities- Series A Convertible Preferred Stock”.
Other Transactions
None.
Director Independence
Messrs. Elsey, Enright and Hanson, three members of our Board of Directors, are independent using the definition of independence under Nasdaq Listing Rule 5605(a)(2) and the standards established by the SEC.
Policies and Procedures for Related Party Transactions
For purposes of our policy only, a related person transaction is a transaction, arrangement or relationship, or any series of similar transactions, arrangements, or relationships, in which we and any related person are, were or will be participants in which the amount involved exceeds the lesser of $120,000 or 1% of the average of our total assets at year-end. Transactions involving compensation for services provided to us as an employee or director are not covered by this policy. A related person is any executive officer, director, or beneficial owner of more than 5% of any class of our voting securities, including any of their immediate family members and any entity owned or controlled by such persons.
| 41 |
Under the policy, if a transaction has been identified as a related person transaction, including any transaction that was not a related person transaction when originally consummated or any transaction that was not initially identified as a related person transaction prior to consummation, our management must present information regarding the related person transaction to our audit committee, or, if audit committee approval would be inappropriate, to another independent body of our Board of Directors, for review, consideration and approval or ratification. The presentation must include a description of, among other things, the material facts, the interests, direct and indirect, of the related persons, the benefits to us of the transaction and whether the transaction is on terms that are comparable to the terms available to or from, as the case may be, an unrelated third party or to or from employees generally. Under the policy, we will collect information that we deem reasonably necessary from each director, executive officer and, to the extent feasible, significant stockholder to enable us to identify any existing or potential related-person transactions and to effectuate the terms of the policy. In addition, under our code of business conduct and ethics, our employees and directors will have an affirmative responsibility to disclose any transaction or relationship that reasonably could be expected to give rise to a conflict of interest. In considering related person transactions, our audit committee, or other independent body of our Board of Directors, will take into account the relevant available facts and circumstances including, but not limited to:
| ● | the risks, costs and benefits to us; | |
| ● | the impact on a director’s independence in the event that the related person is a director, immediate family member of a director or an entity with which a director is affiliated; | |
| ● | the availability of other sources for comparable services or products; and | |
| ● | the terms available to or from, as the case may be, unrelated third parties or to or from employees generally. |
The policy requires that, in determining whether to approve, ratify or reject a related person transaction, our audit committee, or other independent body of our Board of Directors, must consider, in light of known circumstances, whether the transaction is in, or is not inconsistent with, our best interests and those of our stockholders, as our audit committee, or other independent body of our Board of Directors, determines in the good faith exercise of its discretion.
Item 14. Principal Accounting Fees and Services
The following table summarizes the fees billed by M&K CPAs for the fiscal years ended December 31, 2023 and 2022, inclusive of out-of-pocket expenses.
Pre-Approval Policy
Our audit committee was formed upon the consummation of our initial public offering. As a result, the audit committee did not pre-approve all of the foregoing services, although any services rendered prior to the formation of our audit committee were approved by our board of directors. Since the formation of our audit committee, and on a going-forward basis, the audit committee has and will pre-approve all auditing services and permitted non-audit services to be performed for us by our auditors, including the fees and terms thereof (subject to the de minimis exceptions for non-audit services described in the Exchange Act which are approved by the audit committee prior to the completion of the audit).
| Year Ended December 31, | ||||||||
| Fee Category | 2023 | 2022 | ||||||
| Audit fees(1) | $ | 35,200 | $ | 52,450 | ||||
| Audit-related fees(2) | 36,550 | 12,150 | ||||||
| Tax fees(3) | - | - | ||||||
| All other fees(4) | - | - | ||||||
| Total fees | $ | 71,750 | $ | 64,600 | ||||
| (1) | Audit fees consist of fees for professional services rendered in connection with the annual audit of our consolidated financial statements, the review of our quarterly condensed consolidated financial statements and consultations on accounting matters directly related to the audit. |
| (2) | Audit-related fees consist of fees for professional services rendered in connection with the submission of our Registration Statement on Form S-1 in connection with our initial public offering. |
| (3) | Tax fees consist of fees for professional services for tax compliance, tax advice and tax planning. |
| (4) | All other fees consist of fees related to engagement administration. |
| 42 |
PART IV
Item 15. Exhibits, Financial Statement Schedules
| a) | Financial Statements |
For a list of the consolidated financial statements included herein, see Index to Consolidated Financial Statements on page F-1 of this Annual Report, which is incorporated into this Item by reference.
| b) | Exhibits |
| ● | Filed herewith. |
ITEM 16. FORM 10-K SUMMARY
None.
| 43 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| March 29, 2024 | Bullfrog AI Holdings, Inc. | |
| By: | /s/ Vininder Singh | |
| Vininder Singh | ||
| Chief Executive Officer and Director (Principal Executive Officer) | ||
| By: | /s/ Dane Saglio | |
| Dane Saglio | ||
| Chief Financial Officer (Principal Financial and Accounting Officer) | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | |||
| By: | /s/ Vininder Singh | Chief Executive Officer and Chairman | March 29, 2024 | ||
| Vininder Singh | (Principal Executive Officer) | ||||
| By: | /s/ Dane Saglio | Chief Financial Officer | March 29, 2024 | ||
| Dane Saglio | (Principal Financial and Accounting Officer) | ||||
| By: | /s/ Don Elsey | Director | March 29, 2024 | ||
| R. Don Elsey | |||||
| By: | /s/ William Enright | Director | March 29, 2024 | ||
| William Enright | |||||
| By: | /s/ Jason Hanson | Director | March 29, 2024 | ||
| Jason Hanson | |||||
| 44 |
BULLFROG AI HOLDINGS, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Bullfrog AI Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Bullfrog AI Holdings, Inc. (the Company) as of December 31, 2023 and 2022, and the related consolidated statements of operations, changes in stockholders’ deficit, and cash flows for the years ended December 31, 2023 and 2022, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022 and the results of its operations and its cash flows for flows for the two-year period ended December 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of a critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which they relate.
As discussed in Note 2, the Company had a going concern disclosure in the previous year due to continued net losses from operations and negative cash flows in operations. Auditing management’s evaluation of a going concern can be a significant judgment given the fact that the Company uses management estimates on future revenues and expenses, which are difficult to substantiate.
We evaluated the appropriateness of the removal of the going concern, we examined and evaluated the financial information along with management’s plans to mitigate the going concern and management’s disclosure on going concern.
| /s/
|
|
| We have served as the Company’s auditor since 2021. | |
| March 29, 2024 |
| F-2 |
BULLFROG AI HOLDINGS, INC.
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Prepaid expenses | ||||||||
| Total current assets | ||||||||
| Property and equipment, net | ||||||||
| Total assets | $ | $ | ||||||
| Liabilities and Stockholders’ Equity (Deficit) | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | $ | ||||||
| Accrued expenses | ||||||||
| Deferred revenue | ||||||||
| Convertible notes | ||||||||
| Convertible notes - related party | ||||||||
| Total current liabilities | ||||||||
Total liabilities | $ | $ | ||||||
| Stockholders’ equity (deficit): | ||||||||
| Series A Convertible Preferred stock, $ par value, shares authorized; shares issued and outstanding, as of December 31, 2023 and 2022. | ||||||||
| Common stock, $ par value, shares authorized; and shares issued and outstanding as of December 31, 2023 and 2022, respectively. | ||||||||
| Additional paid-in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Total stockholders’ equity (deficit) | ( | ) | ||||||
| Total liabilities and stockholders’ equity (deficit) | $ | $ | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-3 |
BULLFROG AI HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Revenue: | ||||||||
| Revenue, net Revenue | $ | $ | ||||||
| Total revenue | ||||||||
| Cost of goods sold: | ||||||||
| Cost of goods sold | ||||||||
| Total cost of goods sold | ||||||||
| Gross profit | ||||||||
| Operating expenses: | ||||||||
| Research and development | ||||||||
| General and administrative | ||||||||
| Total operating expenses | ||||||||
| Loss from operations | ( | ) | ( | ) | ||||
| Other income (expense), net | ||||||||
| Interest expense, net | ( | ) | ( | ) | ||||
| Loss on conversion of notes | ( | ) | ||||||
| Interest income | ||||||||
| Total other income (expense), net | ( | ) | ||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Net loss per common share attributable to common stockholders - basic and diluted | $ | ) | $ | ) | ||||
| Weighted average number of shares outstanding - basic and diluted | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
BULLFROG AI HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
Series A Preferred Stock | Common Stock | Additional Paid-in | Accumulated | Total Stockholders’ Equity | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | (Deficit) | ||||||||||||||||||||||
| Balance at December 31, 2021 | $ | $ | $ | $ | ( | ) | ( | ) | ||||||||||||||||||||
| Imputed interest | - | - | ||||||||||||||||||||||||||
| Stock-based compensation | - | - | ||||||||||||||||||||||||||
| Reclassification of warrant | - | - | ( | ) | ( | ) | ||||||||||||||||||||||
| Conversion of convertible notes | - | |||||||||||||||||||||||||||
| Shares cancellation | ( | ) | ( | ) | ||||||||||||||||||||||||
| Shares issuance for license | - | |||||||||||||||||||||||||||
| Common stock converted to Series A Preferred Stock | ( | ) | ( | ) | ||||||||||||||||||||||||
| Net loss | - | - | ( | ) | ( | ) | ||||||||||||||||||||||
| Balance at December 31, 2022 | ( | ) | ( | ) | ||||||||||||||||||||||||
| Stock-based compensation | - | - | ||||||||||||||||||||||||||
| Issuance of common stock (initial public offering), net of issuance costs | - | |||||||||||||||||||||||||||
| Issuance of common stock for services | - | |||||||||||||||||||||||||||
| Conversion of convertible debt to common stock | - | |||||||||||||||||||||||||||
| Issuance of common stock pursuant to warrant exercises | - | |||||||||||||||||||||||||||
| Net loss | - | - | ( | ) | ( | ) | ||||||||||||||||||||||
| Balance at December 31, 2023 | $ | $ | $ | $ | ( | ) | $ | |||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
BULLFROG AI HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | ||||||||
| Stock-based compensation | ||||||||
| Shares issued for license | ||||||||
| Shares issued for services | ||||||||
| Loss on conversion of notes | ||||||||
| Amortization of debt discount | ||||||||
| Imputed interest | ||||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expense | ( | ) | ( | ) | ||||
| Accounts payable | ( | ) | ||||||
| Accrued expenses | ( | ) | ||||||
| Accrued expenses - related party | ||||||||
| Deferred revenue | ( | ) | ||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchases of property and equipment | ( | ) | ||||||
| Net cash used in investing activities | ( | ) | ||||||
| Cash flows from financing activities: | ||||||||
| Proceeds from issuance of common stock (initial public offering), net of issuance costs | ||||||||
| Proceeds from exercise of warrants | ||||||||
| Proceeds from convertible notes payable | ||||||||
| Proceeds from notes payable | ||||||||
| Payments of notes payable | ( | ) | ||||||
| Repayment of note payable and interest - related party | ( | ) | ||||||
| Proceeds from short term insurance financing | ||||||||
| Payments of short term insurance financing | ( | ) | ||||||
| Net cash provided by financing activities | ||||||||
| Net increase in cash and cash equivalents | ||||||||
| Cash and cash equivalents, beginning of period | ||||||||
| Cash and cash equivalents, end of period | $ | $ | ||||||
| Supplemental cash flow information: | ||||||||
| Cash paid for interest | $ | $ | ||||||
| Cash paid for taxes | ||||||||
| Supplemental non-cash activity | ||||||||
| Reclassification of warrant | $ | $ | ||||||
| Issuance of common stock upon conversion of notes payable | $ | $ | ||||||
| Conversion of convertible note payable | $ | $ | ||||||
| Cancellation of common stock | $ | $ | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
BULLFROG AI HOLDINGS, INC.
NOTES TO FINANCIAL STATEMENTS
December 31, 2023 and 2022
1. Organization and Nature of Business
Description of Business
Bullfrog AI Holdings, Inc. (“we”, “our” or the “Company”) was incorporated in the State of Nevada on February 6, 2020. Bullfrog AI Holdings, Inc. is the parent company of Bullfrog AI, Inc. and Bullfrog AI Management, LLC which were incorporated in Delaware and Maryland, in 2017 and 2021, respectively. All of our operations are currently conducted through BullFrog AI Holdings, Inc., which began operations on February 6, 2020. We are a company focused specifically on advanced AI/ML-driven analysis of complex data sets in medicine and healthcare. Our objective is to utilize our platform for precision medicine approach to drug asset enablement through external partnerships and selective internal development.
Most new therapeutics will fail at some point in preclinical or clinical development. This is the primary driver of the high cost of developing new therapeutics. A major part of the difficulty in developing new therapeutics is efficient integration of complex and highly dimensional data generated at each stage of development to de-risk subsequent stages of the development process. Artificial Intelligence and Machine Learning (AI/ML) has emerged as a digital solution to help address this problem.
We use artificial intelligence and machine learning to advance medicines for both internal and external projects. Most current AI/ML platforms still fall short in their ability to synthesize disparate, high-dimensional data for actionable insight. Our platform technology, named, bfLEAP™ is an analytical AI/ML platform developed at The Johns Hopkins University Applied Physics Laboratory (JHU-APL) which is able to surmount the challenges of scalability and flexibility currently hindering researchers and clinicians by providing a more precise, multi-dimensional understanding of their data. We are deploying bfLEAP™ for use at several critical stages of development for internal programs and through strategic partnerships and collaborations with the intention of streamlining data analytics in therapeutics development, decreasing the overall development costs by decreasing failure rates for new therapeutics, and impacting the lives of countless patients that may otherwise not receive the therapies they need.
The bfLEAP™ platform utilizes both supervised and unsupervised machine learning – as such, it is able to reveal real/meaningful connections in the data without the need for a priori hypothesis. Algorithms used in the bfLEAP™ platform are designed to handle highly imbalanced data sets to successfully identify combinations of factors that are associated with outcomes of interest.
Our primary goal is to improve the odds of success at any stage of pre-clinical and clinical therapeutics development, for in house programs, and our strategic partners and collaborators. Our primary business model is enabling the success of ongoing clinical trials or rescue of late stage failed drugs (i.e., Phase 2 or Phase 3 clinical trial failures) for development and divestiture; although, we will also consider collaborations for earlier stage drugs. We hope to accomplish this through strategic acquisitions of current clinical stage and failed drugs for in-house development, or through strategic partnerships with biopharmaceutical industry companies. We are able to pursue our drug asset enhancement business by leveraging a powerful and proven AI/ML platform (trade name: bfLEAP™) initially developed at JHU-APL. We believe the bfLEAP™ analytics platform is a potentially disruptive tool for analysis of pre-clinical and/or clinical data sets, such as the robust pre-clinical and clinical trial data sets being generated in translational R&D and clinical trial settings.
Liquidity and Going Concern
The
Company has had negative cash flows from operations and operated at a net loss since inception. In the first quarter of 2023, we completed
our initial public offering (“IPO”). In February 2024 the Company received net proceeds of approximately $
| F-7 |
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements include the accounts of Bullfrog AI Holdings, Inc. and our wholly owned subsidiaries and have been prepared in conformity with United States generally accepted accounting principles (“GAAP”). All intercompany accounts and transactions have been eliminated in consolidation.
On
February 13, 2023, we
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires us to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. These estimates include, but are not limited to, revenue recognition, allowances for doubtful accounts, recoverability of deferred tax assets and certain other of our accrued liabilities. Actual results could differ from these estimates.
Revenue Recognition
The Company recognizes revenue based on the following five step model:
| ● | Identification of the contract with a customer |
This step outlines the criteria that must be met when establishing a contract with a customer to supply goods or services.
| ● | Identification of the performance obligations in the contract |
This step describes how distinct performance obligations in the contract must be handled.
| ● | Determination of the transaction price |
This step outlines what must be considered when establishing the transaction price, which is the amount the business expects to receive for transferring the goods and services to the customer.
| ● | Allocation of the transaction price to the performance obligations in the contract |
This step outlines guidelines for allocating the transaction price across the contract’s separate performance obligations, and is what the customer agrees to pay for the goods and services.
| ● | Recognition of revenue when, or as, the Company satisfies a performance obligation |
Revenue can be recognized as the business meets each performance obligation. This step specifies how that should happen.
Contract Services
The Company anticipates that the majority of revenues to be recognized in the near future will result from our fee for service partnership offering, designed for biopharmaceutical companies, as well as other organizations, of all sizes that have challenges analyzing data throughout the drug development process. The Company provides the customer with an analysis of large complex data sets using the Company’s proprietary Artificial Intelligence / Machine Learning platform called bfLEAP™. This platform is designed to predict targets of interest, patterns, relationships, and anomalies. The Company believes that there will be additional on-going work requested from partners therefore the service model utilizes a master services agreement with work or task orders issued for discrete analysis performed at the discovery, preclinical, or clinical stages of drug development. The Company receives a cash fee and in some instances the potential for rights to new intellectual property generated from the analysis. Once data analysis and the analysis report are complete, the Company delivers the analysis set to the customer and recognizes revenue at that point in time.
| F-8 |
Financial Instruments
The carrying value of short-term instruments, including cash and cash equivalents, accounts payable and accrued expenses approximate fair value due to the relatively short period to maturity for these instruments.
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value maximize the use of observable inputs and minimize the use of unobservable inputs. The Company utilizes a three-level valuation hierarchy for disclosures of fair value measurements, defined as follows:
Level 1 - inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level 2 - inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments.
Level 3 - inputs to the valuation methodology are unobservable and significant to the fair value. The Company does not have any assets or liabilities that are required to be measured and recorded at fair value on a recurring basis.
Cash
The
Company considers cash to consist of cash on hand and temporary investments having an original maturity of 90 days or less that are readily
convertible into cash. As of December 31, 2023 and 2022, cash balances were $
Concentrations of Credit Risk
The Company’s financial instruments that are exposed to a concentration of credit risk are cash and accounts receivable. Occasionally, the Company’s cash in interest-bearing accounts may exceed FDIC insurance limits. The financial stability of these institutions is periodically reviewed by senior management.
Cost of Sales
Cost of sales is comprised of royalties and the cost of outsourced services provided to the Company related to customer service contracts.
Property and Equipment
Property and equipment are stated at cost. When retired or otherwise disposed, the related carrying value and accumulated depreciation are removed from the respective accounts and the net difference less any amount realized from disposition is reflected in earnings. For financial statement purposes, property and equipment are recorded at cost and depreciated using the straight-line method over their estimated useful lives.
Advertising
The Company follows the policy of charging the costs of advertising to expense as incurred.
Income Taxes
Deferred income tax assets and liabilities are determined based on the estimated future tax effects of net operating loss and credit carry forwards and temporary differences between the tax basis of assets and liabilities and their respective financial reporting amounts measured at the current enacted tax rates. The Company records an estimated valuation allowance on its deferred income tax assets if it is not more likely than not that these deferred income tax assets will be realized.
The Company recognizes a tax benefit from an uncertain tax position if it is more likely than not that the tax position will be sustained on examination by taxing authorities. Interest and penalties associated with such uncertain tax positions are classified as a component of income tax expense.
| F-9 |
Stock-Based Compensation
Employee and non-employee share-based compensation is measured at the grant date, based on the fair value of the award, and is recognized as an expense over the requisite service period.
We calculate basic net loss per share by dividing the net loss by the weighted-average number of shares of common stock outstanding during the period.
Diluted earnings per share is computed by giving effect to all potentially dilutive common stock equivalents in the period, including unvested stock options and warrants. As we have reported losses for all periods presented, all potentially dilutive securities have been excluded from the calculation of diluted net loss per share as their effect would be antidilutive.
Recent Accounting Pronouncements
In December 2023, the FASB issued ASU No. 2024-09: Income Taxes (Topic 740): Improvements to Income Tax Disclosures that requires entities to disclose additional information about federal, state, and foreign income taxes primarily related to the income tax rate reconciliation and income taxes paid. The new standard also eliminates certain existing disclosure requirements related to uncertain tax positions and unrecognized deferred tax liabilities. The guidance is effective for our fiscal year ending December 31, 2025. The guidance does not affect recognition or measurement in our consolidated financial statements.
The Company does not believe that any other recently issued effective pronouncements, or pronouncements issued but not yet effective, if adopted, would have a material effect on the accompanying financial statements.
3. Property and Equipment
Property
and equipment consisted of $
Depreciation
expense totaled $
4. Convertible Notes
March 2020 Note
On
March 27, 2020, the Company entered into a convertible loan agreement with the Maryland Technology Development Corporation with a principal
balance of $
August 2021 Note
In August 2021, the Company entered into a convertible loan agreement with an unrelated party for a commitment of up to $ with a % original issue discount and a % interest rate. The loan provided for a maturity date of . We borrowed $ and $ of principal in the years ended December 31, 2021 and 2022, respectively. The noteholder had the right to convert the principal and interest into common shares of the Company at the IPO at a% discount to the IPO price.
| F-10 |
As of December 31, 2022, the loan was outstanding with a principal balance of $ and accrued interest of $. The loan was paid in its entirety in February 2023.
In connection with the convertible loan agreement, the Company also issued Warrants with an exercise price of $ exercisable for from issuance. In May 2022, the Company and the note holder agreed to cancel and void the warrants and enter into a new agreement for warrants with an exercise price of $. The Company assessed the differences in fair value and determined that they were de minimis and expensed the full value of the new warrants.
December 2021 Note
On December 20, 2021, the Company entered into a loan agreement with an unrelated party. The loan provided for a maturity, a % original issue discount and a % interest rate. The Company received $ of proceeds from this note.
The note was automatically convertible into shares of common stock at a discount to the IPO price or based on the valuation of the Company, whichever was more favorable to the holder.
Initially, the loan was estimated to be issued with warrants. Subsequent to the closing of the loan agreement, the Company enhanced the terms of the Bridge Note Offering under which the loan was closed and in April 2022 closed on the sale of approximately $ million in face value of convertible bridge notes. Pursuant to the enhanced terms, the warrants were issued concurrently with the conversion of the note.
Concurrent with the closing of the Company’s IPO, the note converted according to its terms into shares of common stock. No gain or loss was recognized on the conversion.
Convertible Bridge Notes
On
April 11, 2022, the Company entered into an Exclusive placement agent and/or underwriter agreement with WallachBeth Capital LLC in connection
with a proposed private and/or public offerings by the Company. On April 28, 2022, the Company received approximately $
The
Convertible Bridge Notes were initially convertible at the IPO at a
In
connection with the Convertible Bridge Notes, the purchasers were also entitled to conditional warrants to be issued upon completion
of the Company’s IPO.
In the fourth quarter of 2022, .
Concurrent with the closing of the Company’s IPO in February 2023, all of the Convertible Bridge Notes converted according to their terms into shares of common stock. No gain or loss was recognized on the conversion.
| F-11 |
5. Convertible Notes – Related Party
SAFE Agreement
On
July 8, 2021, the Company entered into a Simple Agreement for Future Equity (SAFE), with a related party, at a purchase price of $
In
February 2023, the SAFE terminated and converted into shares of common stock according to its terms upon the Company’s closing
of its IPO. The conversion was considered a redemption for accounting purposes and consequently, the Company recognized a $
As
of December 31, 2022, the $
August 2021 Note
On
August 19, 2021, the Company entered into a convertible loan agreement with a related party, with a principal balance of $
In
February 2023, the related party elected to convert the convertible loan into shares of common stock according to its terms upon
the Company’s closing of its IPO. The conversion was considered a redemption for accounting purposes and consequently, the Company
recognized a $
In
connection with the convertible loan agreement, the Company also issued
6. Related Party
During the year ended December 31, 2023, the Company issued stock options to its Chief Financial Officer for services rendered.
During the year ended December 31, 2021, the Company issued common stock options to related parties for services rendered. The options have an original life of years and . During the years ended December 31, 2023 and 2022, the Company recognized $ and $, respectively of stock-based compensation related to these options.
At various times in 2021, the Company entered into unsecured short term loan agreements with a related party for
an aggregate principal balance of $
7. Notes Payable
In
January 2023 the Company entered into a short-term note payable with a principal balance of $
In
February 2023, the Company entered into an agreement to finance a portion of the premium for its Directors and Officers Insurance. The
agreement provides for financing of $
8. Stockholders’ Equity
Preferred Stock
The
Company has shares of preferred stock authorized at a par value of $ with being designated as Series A Convertible
Preferred Stock. On October 5, 2022, the Company entered into an exchange agreement with an Investor providing for the exchange of
shares of commons stock into shares of Series A Convertible Preferred Stock. Each share of Series A Convertible Preferred Stock
is convertible at any time into shares of the Company’s common stock. The Series A Preferred Stock is the economic equivalent
of the common stock but has no voting rights and is subject to a blocker which prohibits the conversion into common stock if it would
result in the Investor owning more than
| F-12 |
Common Stock
The Company has shares of common stock authorized at a par value of $. During the year ended December 31, 2022, the Company:
| ● | Exchanged shares of common stock for shares of Series A Convertible Preferred stock as noted above, | |
| ● | Issued
shares of common stock pursuant to a conversion of $ | |
| ● | Cancelled shares of common stock as the change in number of shares issued as part of the cancellation of the prior agreements and new agreements with advisors, and | |
| ● | Issued shares of common stock pursuant to a license agreement valued at $189,828. |
After the Company signed two licenses for two drug programs from universities in the first half of 2022 it engaged an independent valuation firm to perform an Enterprise-Equity valuation. The results of this engagement resulted in an increase in the value per share of common stock used in the Black Scholes option pricing model employed to value the Company’s equity grants and warrant issuances.
In
February 2023, the Company completed its IPO for the sale of units (each, a “Unit,” collectively, the “Units”)
at a price of $ per Unit for a total of approximately $
In connection with the completion of its IPO, the Company issued an aggregate of shares of common stock upon the conversion of certain outstanding convertible debt.
In
connection with the IPO, in February 2023, the Company
In
February 2023, the Company issued shares of common stock for consulting services and recognized $
In
the second quarter of 2023, we issued shares of common stock following the exercise of warrants for proceeds of $
Dilutive securities are excluded from the diluted earnings per share calculation because their effect is anti-dilutive. As of December 31, 2023 and December 31, 2022, and warrants were not included in the calculation of net loss per share, respectively. In addition, and options for common shares were not included in the calculation of net loss per share, respectively.
2022 Equity Incentive Plan
In November 2022, the Company’s Board of Directors adopted, and its shareholders approved the 2022 Equity Incentive Plan (the “Plan”). The Plan provides for the granting of equity-based awards to employees, directors, and consultants. The Plan provides for equity-based awards including incentive stock options, non-qualified stock options, stock appreciation rights, performance share awards, cash awards and other equity-based awards. Awards are limited to a maximum term of years and any exercise prices shall not be less than 100% of the fair market value of one share of common stock on the grant date. The Plan authorizes an initial maximum number of shares underlying awards of with an automatic annual % increase beginning in 2024. As of December 31, 2023, there were awards authorized but unissued available under the Plan.
| F-13 |
Stock Options
| Number of Shares | Weighted-Average Exercise Price | Weighted-Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value | |||||||||||||
| Outstanding at December 31, 2021 | $ | $ | ||||||||||||||
| Granted | $ | |||||||||||||||
| Exercised | $ | |||||||||||||||
| Forfeited / canceled | ( | ) | $ | |||||||||||||
| Outstanding at December 31, 2022 | $ | $ | ||||||||||||||
| Granted | $ | |||||||||||||||
| Exercised | $ | |||||||||||||||
| Forfeited / canceled | $ | |||||||||||||||
| Outstanding at December 31, 2023 | $ | $ | ||||||||||||||
| Vested at December 31, 2023 | $ | $ | ||||||||||||||
| 2023 | ||||
| Expected dividend yield | ||||
| Expected volatility | % - % | |||
| Risk-free interest rate | % - % | |||
| Expected life (in years) | - | |||
| ● | Volatility - The trading volatility was determined by calculating the volatility of the Company’s peer group. | |
| ● | Expected life of options – The expected life of options granted to employees was determined using the simplified method. | |
| ● | Risk-free interest rate – This is the U.S. Treasury rate, having a term comparable to the expected life of the stock option. | |
| ● | Dividend yield – The Company does not expect to pay a dividend in the foreseeable future. |
The weighted-average grant-date fair value of options granted during the year ended December 31, 2023 was $. The total grant-date fair value of options granted and vested during the year ended December 31, 2023 was approximately $ and $, respectively.
options were exercised in any of the periods presented.
During
the years ended December 31, 2023 and 2022, the Company recognized $
As of December 31, 2023, the total unrecognized compensation expense related to unvested stock options, was approximately $, which the Company expects to recognize over a weighted-average period of approximately years.
Warrants
During
the years ended December 31, 2023 and 2022, the Company granted a total of and warrants, respectively. The warrants
have an original life of and . During the year ended December
31, 2023, warrants to purchase shares vested and had a fair value of $. During the year ended December 31, 2022,
shares of warrants vested and amended with a fair value of $,
| F-14 |
During
the year ended December 31, 2021, the Company granted a total of warrants. Of this amount, warrants, with a fair value
of $
During
the year ended December 31, 2021, the Company issued
The
During the year ended December 31, 2023, the Company issued the following warrants:
| ● | In
February 2023, in connection with the completion of the initial public offering, the Company
issued |
| ● | In
February 2023, in connection with the completion of the initial public offering, the Company
issued |
| ● | As
part of the sale of units in the Company’s initial public offering the Company issued
|
| ● | In
February 2023, as part of the Company’s initial public offering, the Company issued
|
| F-15 |
The following table provides details over the Company’s outstanding warrants including those issued as consideration for services and those issued in conjunction with transactions as of December 31, 2023:
| Exercise Price | Expiration | Number of Warrants | ||
| $ | ||||
| $ | ||||
| $ | ||||
| $ | ||||
| $ | ||||
During the years ended December 31, 2023 and 2022, the Company recognized $ and $, respectively of compensation expense related to certain warrants.
Warrants Issued as Consideration for Services
The following table summarizes the activity for warrants issued as consideration for services for the years ended December 31, 2023 and 2022:
| Number of Warrants | Weighted-Average Exercise Price | Weighted-Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value | |||||||||||||
| Outstanding at December 31, 2021 | $ | | $ | |||||||||||||
| Granted | $ | |||||||||||||||
| Exercised | $ | |||||||||||||||
| Forfeited / canceled | ( | ) | $ | |||||||||||||
| Outstanding at December 31, 2022 | $ | $ | ||||||||||||||
| Granted | $ | |||||||||||||||
| Exercised | $ | |||||||||||||||
| Forfeited / canceled | $ | |||||||||||||||
| Outstanding at December 31, 2023 | $ | $ | ||||||||||||||
| Vested at December 31, 2023 | $ | $ | ||||||||||||||
| 2022 | ||||
| Expected dividend yield | % | |||
| Expected volatility | % | |||
| Risk-free interest rate | % - % | |||
| Expected life (in years) | ||||
| ● | Volatility - The trading volatility was determined by calculating the volatility of the Company’s peer group. | |
| ● | Expected life of options – The expected life of options granted to employees was determined using the simplified method. | |
| ● | Risk-free interest rate – This is the U.S. Treasury rate, having a term comparable to the expected life of the stock option. | |
| ● | Dividend yield – The Company does not expect to pay a dividend in the foreseeable future. |
No warrants were issued in the year ended December 31, 2023.
As of December 31, 2023, the total unrecognized compensation expense related to unvested warrants was approximately $, which the Company expects to recognize over a weighted-average period of approximately years.
The total grant-date fair value of warrants vested during the year ended December 31, 2023 was approximately $.
| F-16 |
9. Income Taxes
Deferred income taxes reflect the net tax effects of loss and credit carryforwards and temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of our deferred tax assets for federal and state income taxes are as follows:
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Deferred tax assets: | ||||||||
| Net operating losses | $ | $ | ||||||
| Capitalized research and development | ||||||||
| Stock-based compensation | ||||||||
| Intangibles | ||||||||
| Other | ||||||||
| Total deferred tax assets | ||||||||
| Valuation allowance | ( | ) | ( | ) | ||||
| Net deferred tax asset | ||||||||
| Deferred tax liabilities: | ||||||||
| Property and equipment | ( | ) | ||||||
| Total deferred tax liabilities | ( | ) | ||||||
| Net deferred tax asset / (liability) | $ | $ | ||||||
Realization
of our deferred tax assets is dependent upon future earnings, if any, the timing, and amount of which are uncertain. Because of our lack
of U.S. earnings history, the net U.S. deferred tax assets have been fully offset by a valuation allowance. The valuation allowance increased
by $
As
of December 31, 2023, the Company has available for federal income tax purposes a net operating loss carry forward of approximately $
The Company has provided a valuation reserve against the full amount of the net operating loss benefit, since in the opinion of management, based upon the earnings history of the Company; it is more likely than not that the benefits will not be realized. All or portion of the remaining valuation allowance may be reduced in future years based on an assessment of earnings sufficient to fully utilize these potential tax benefits.
We have incurred net operating losses since inception and we do not have any significant unrecognized tax benefits. Our policy is to include interest and penalties related to unrecognized tax benefits, if any, within the provision for taxes in the consolidated statements of operations. If we are eventually able to recognize our uncertain positions, our effective tax rate would be reduced. We currently have a full valuation allowance against out net deferred tax asset which would impact the timing of the effective tax rate benefit should any of these uncertain tax positions be favorably settled in the future. Any adjustments to our uncertain tax positions would result in an adjustment of our net operating loss or tax credit carry forwards rather than resulting in a cash outlay.
We file income tax returns in the U.S. and certain state jurisdictions. We are not currently under examination in these jurisdictions for any tax year. The Company’s tax years beginning with 2020 are open tax years. Because of net operating losses and research credit carryovers, substantially all of our tax years remain open to examination.
| F-17 |
The Company did not have unrecognized tax benefits as of December 31, 2023 and 2022, and does not anticipate this to change significantly over the next 12 months. The Company will recognize interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense. Reconciliations between the statutory federal income tax rate and the effective income tax rate of income tax expense is as follows:
| December 31, | ||||
| 2023 | ||||
| U.S. Federal statutory tax rate | % | |||
| Stock-based compensation | ) | |||
| Other | ( | ) | ||
| Change in valuation allowance | ( | ) | ||
| % | ||||
10. Material Agreements
JHU-APL Technology License
On
February 7, 2018, the Company entered into an exclusive, world-wide, royalty-bearing license from JHU-APL for the technology. The license
covers three (3) issued patents, one (1) new provisional patent application, non-patent rights to proprietary libraries of algorithms
and other trade secrets, the license also includes modifications and improvements. In October of 2021, the Company executed an amendment
to the original license which represents improvements and new advanced analytics capabilities. In consideration of the rights granted
to the Company under the License Agreement JHU received a warrant equal to five percent (
On
May 31, 2023, the Company and JHU-APL entered into Amendment number 1 of the July 8, 2022 License Agreement whereby the Company gained
access to certain improvements including additional patents and knowhow in exchange for a series of payments totaling $
| F-18 |
George Washington University - Beta2-spectrin siRNA License
On January 14, 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from George Washington University (GWU) for rights to use siRNA targeting Beta2-spectrin in the treatment of human diseases, including hepatocellular carcinoma (HCC). The license covers methods claimed in three US and worldwide patent applications, and also includes use of this approach for treatment of obesity, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis.
In
consideration of the rights granted to the Company under the License Agreement the Company paid GWU a $
Johns Hopkins University – Mebendazole License
On February 22, 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from Johns Hopkins University (JHU) for the use of an improved formulation of Mebendazole for the treatment of any human cancer or neoplastic disease. This formulation shows potent activity in animal models of different types of cancer and has been evaluated in a Phase I clinical trial in patients with high-grade glioma (NCT01729260). The trial, an open-label dose-escalation study, assessed the safety and efficacy of the improved formulation with adjuvant temozolomide in 24 patients with newly diagnosed gliomas. Investigators observed no dose-limiting toxicity in patients receiving all but the highest tested dose (200mg/kg/day). Four of the 15 patients receiving the maximum tested dose of 200mg/kg/day experienced dose-limiting toxicity, all of which were reversed by decreasing or eliminating the dose given. There were no serious adverse events attributed to Mebendazole at any dose during the trial. 41.7% of patients who received Mebendazole were alive at two years after enrollment, and 25% were alive at four years (Gallia et al., 2021).
The
license covers six (6) issued patents and one (1) pending application. In consideration of the rights granted to the Company under the
License Agreement, JHU will receive a staggered Upfront License Fee of $
| F-19 |
Johns Hopkins University – Prodrug License
On
October 13, 2022, the Company entered into an exclusive, world-wide, royalty-bearing license from JHU and the Institute of Organic Chemistry
and Biochemistry (IOCB) of the Czech Academy of Sciences for rights to commercialize N-substituted prodrugs of Mebendazole that demonstrate
improved solubility and bioavailability. The license covers prodrug compositions and use for treating disease as claimed in multiple
US and worldwide patent applications. In consideration for the rights granted to the Company under the License Agreement JHU and IOCB
will receive a staggered upfront license fee of $
11. Commitments and Contingencies
While not assured, management does not believe, based upon information available at this time, that a loss contingency will have a material adverse effect on the Company’s financial position, results of operations or cash flows. Additionally, the Company does not have any material commitments.
12. Subsequent Events
On
February 5, 2024 the Company received net proceeds of approximately $
| F-20 |